AKASH GOYAL AskiitiansExpert-IITD
Last Activity: 14 Years ago
Dear Pranathi
Electrophilic Substitution of Disubstituted Benzene Rings
When a benzene ring has two substituent groups, each exerts an influence on subsequent substitution reactions. The activation or deactivation of the ring can be predicted more or less by the sum of the individual effects of these substituents. The site at which a new substituent is introduced depends on the orientation of the existing groups and their individual directing effects. We can identify two general behavior categories, as shown in the following table. Thus, the groups may be oriented in such a manner that their directing influences act in concert, reinforcing the outcome; or are opposed (antagonistic) to each other. Note that the orientations in each category change depending on whether the groups have similar or opposite individual directing effects.
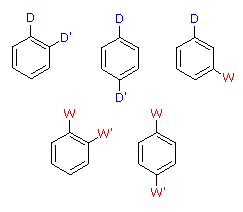
Orientational Interaction of Substituents
|
|
Antagonistic or Non-Cooperative
|
|
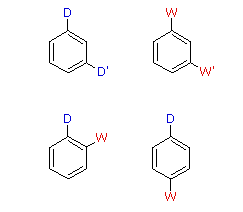
Reinforcing or Cooperative
|
 |
|
 |
|
D = Electron Donating Group (ortho/para-directing)
W = Electron Withdrawing Group (meta-directing)
|
|
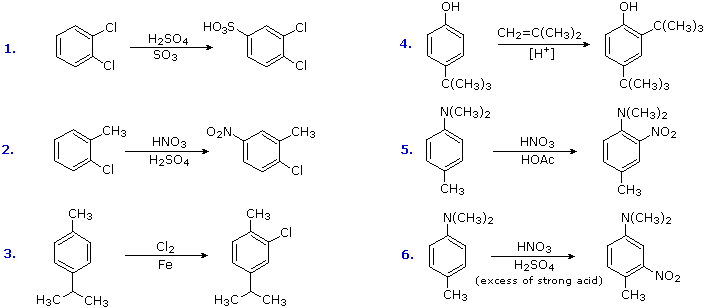
The products from substitution reactions of compounds having a reinforcing orientation of substituents are easier to predict than those having antagonistic substituents. For example, the six equations shown below are all examples of reinforcing or cooperative directing effects operating in the expected manner. Symmetry, as in the first two cases, makes it easy to predict the site at which substitution is likely to occur. Note that if two different sites are favored, substitution will usually occur at the one that is least hindered by ortho groups.
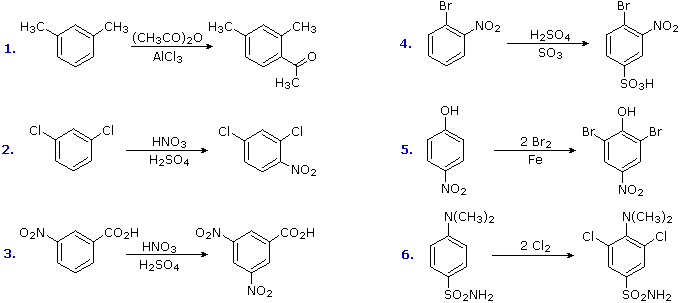
The first three examples have two similar directing groups in a meta-relationship to each other. In examples 4 through 6, oppositely directing groups have an ortho or para-relationship. The major products of electrophilic substitution, as shown, are the sum of the individual group effects. The strongly activating hydroxyl (–OH) and amino (–NH2) substituents favor dihalogenation in examples 5 and six.
Substitution reactions of compounds having an antagonistic orientation of substituents require a more careful analysis. If the substituents are identical, as in example 1 below, the symmetry of the molecule will again simplify the decision. When one substituent has a pair of non-bonding electrons available for adjacent charge stabilization, it will normally exert the product determining influence, examples 2, 4 & 5, even though it may be overall deactivating (case 2). Case 3 reflects a combination of steric hindrance and the superior innate stabilizing ability of methyl groups relative to other alkyl substituents. Example 6 is interesting in that it demonstrates the conversion of an activating ortho/para-directing group into a deactivating meta-directing "onium" cation [–NH(CH3)2(+) ] in a strong acid environment.
Nucleophilic Substitution, Elimination & Addition Reactions of Benzene Derivatives
1. Substitution
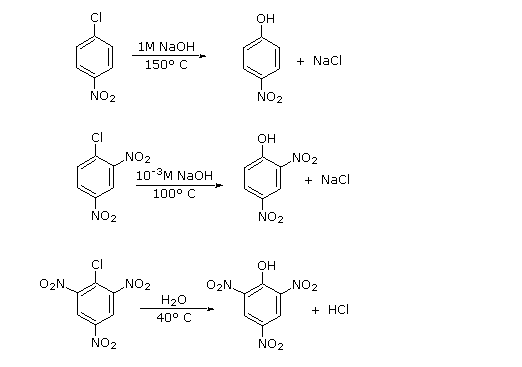
An early method of preparing phenol (the Dow process) involved the reaction of chlorobenzene with a concentrated sodium hydroxide solution at temperatures above 350 ºC. The chief products are phenol and diphenyl ether (see below). This apparent nucleophilic substitution reaction is surprising, since aryl halides are generally incapable of reacting by either an SN1 or SN2 pathway.
C6H5–Cl + NaOH solution |
350 ºC
 |
C6H5–OH + C6H5–O–C6H5 + NaCl |
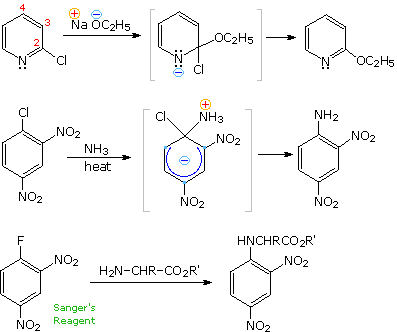
The presence of electron-withdrawing groups (such as nitro) ortho and para to the chlorine substantially enhance the rate of substitution, as shown in the set of equations presented on the left below. To explain this, a third mechanism for nucleophilic substitution has been proposed. This two-step mechanism is characterized by initial addition of the nucleophile (hydroxide ion or water) to the aromatic ring, followed by loss of a halide anion from the negatively charged intermediate. This is illustrated by clicking the "Show Mechanism" button next to the diagram. The sites over which the negative charge is delocalized are colored blue, and the ability of nitro, and other electron withdrawing, groups to stabilize adjacent negative charge accounts for their rate enhancing influence at the ortho and para locations.
Three additional examples of aryl halide nucleophilic substitution are presented on the right. Only the 2- and 4-chloropyridine isomers undergo rapid substitution, the 3-chloro isomer is relatively unreactive. Nitrogen nucleophiles will also react, as evidenced by the use of Sanger's reagent for the derivatization of amino acids. The resulting N-2,4-dinitrophenyl derivatives are bright yellow crystalline compounds that facilitated analysis of peptides and proteins, a subject for which Frederick Sanger received one of his two Nobel Prizes in chemistry.
|
Additional Examples
|
All the best.
AKASH GOYAL
AskiitiansExpert-IITD
Please feel free to post as many doubts on our discussion forum as you can. We are all IITians and here to help you in your IIT JEE preparation.
Win exciting gifts by answering the questions on Discussion Forum. So help discuss any query on askiitians forum and become an Elite Expert League askiitian.
Now you score 5+15 POINTS by uploading your Pic and Downloading the Askiitians Toolbar respectively : Click here to download the toolbar..