AKASH GOYAL AskiitiansExpert-IITD
Last Activity: 14 Years ago
Dear Anand
Cycloalkanes
Cycloalkanes have one or more rings of carbon atoms. The simplest examples of this class consist of a single, unsubstituted carbon ring, and these form a homologous series similar to the unbranched alkanes. The IUPAC names of the first five members of this series are given in the following table. The last (yellow shaded) column gives the general formula for a cycloalkane of any size. If a simple unbranched alkane is converted to a cycloalkane two hydrogen atoms, one from each end of the chain, must be lost. Hence the general formula for a cycloalkane composed of n carbons is CnH2n. Although a cycloalkane has two fewer hydrogens than the equivalent alkane, each carbon is bonded to four other atoms so such compounds are still considered to be saturated with hydrogen.
Examples of Simple Cycloalkanes
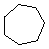
| Name | Cyclopropane | Cyclobutane | Cyclopentane | Cyclohexane | Cycloheptane | Cycloalkane |
Molecular
Formula |
C3H6 |
C4H8 |
C5H10 |
C6H12 |
C7H14 |
CnH2n |
Structural
Formula |
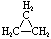 |
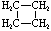 |
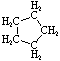 |
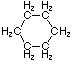 |
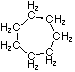 |
(CH2)n |
Line
Formula |
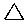 |
 |
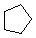 |
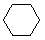 |
 |
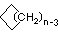 |
Substituted cycloalkanes are named in a fashion very similar to that used for naming branched alkanes. The chief difference in the rules and procedures occurs in the numbering system. Since all the carbons of a ring are equivalent (a ring has no ends like a chain does), the numbering starts at a substituted ring atom.
IUPAC Rules for Cycloalkane Nomenclature
1. For a monosubstituted cycloalkane the ring supplies the root name (table above) and the substituent group is named as usual. A location number is unnecessary.
2. If the alkyl substituent is large and/or complex, the ring may be named as a substituent group on an alkane.
3. If two different substituents are present on the ring, they are listed in alphabetical order, and the first cited substituent is assigned to carbon #1. The numbering of ring carbons then continues in a direction (clockwise or counter-clockwise) that affords the second substituent the lower possible location number.
4. If several substituents are present on the ring, they are listed in alphabetical order. Location numbers are assigned to the substituents so that one of them is at carbon #1 and the other locations have the lowest possible numbers, counting in either a clockwise or counter-clockwise direction.
5. The name is assembled, listing groups in alphabetical order and giving each group (if there are two or more) a location number. The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing.
Small rings, such as three and four membered rings, have significant angle strain resulting from the distortion of the sp3 carbon bond angles from the ideal 109.5º to 60º and 90º respectively. This angle strain often enhances the chemical reactivity of such compounds, leading to ring cleavage products. It is also important to recognize that, with the exception of cyclopropane, cycloalkyl rings are not planar (flat).
Hydrocarbons having more than one ring are common, and are referred to as bicyclic (two rings), tricyclic (three rings) and in general, polycyclic compounds. The molecular formulas of such compounds have H/C ratios that decrease with the number of rings. In general, for a hydrocarbon composed of n carbon atoms associated with m rings the formula is: CnH(2n + 2 - 2m). The structural relationship of rings in a polycyclic compound can vary. They may be separate and independent, or they may share one or two common atoms. Some examples of these possible arrangements are shown in the following table.
Examples of Isomeric C8H14 Bicycloalkanes
| Isolated Rings | Spiro Rings | Fused Rings | Bridged Rings |
| No common atoms |
One common atom |
One common bond |
Two common atoms |
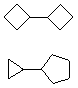 | 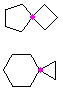 | 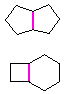 | 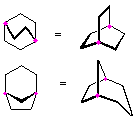 |
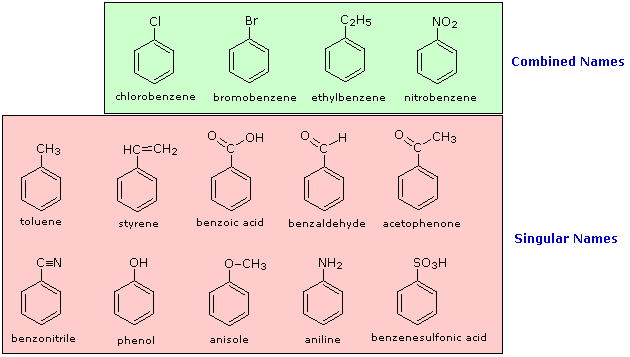
Benzene Derivatives
The nomenclature of substituted benzene ring compounds is less systematic than that of the alkanes, alkenes and alkynes. A few mono-substituted compounds are named by using a group name as a prefix to "benzene", as shown by the combined names listed below. A majority of these compounds, however, are referred to by singular names that are unique. There is no simple alternative to memorization in mastering these names.
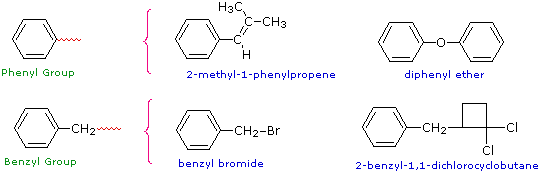
Two commonly encountered substituent groups that incorporate a benzene ring are phenyl, abbreviated Ph-, and benzyl, abbreviated Bn-. These are shown here with examples of their use. Be careful not to confuse a phenyl (pronounced fenyl) group with the compound phenol (pronounced feenol). A general and useful generic notation that complements the use of R- for an alkyl group is Ar- for an aryl group (any aromatic ring).
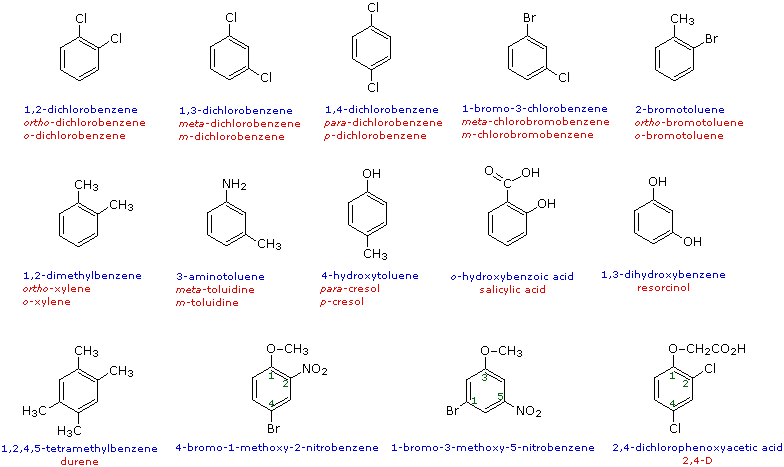
When more than one substituent is present on a benzene ring, the relative locations of the substituents must be designated by numbering the ring carbons or by some other notation. In the case of disubstituted benzenes, the prefixes ortho, meta & para are commonly used to indicate a 1,2- or 1,3- or 1,4- relationship respectively. In the following examples, the first row of compounds show this usage in red. Some disubstituted toluenes have singular names (e.g. xylene, cresol & toluidine) and their isomers are normally designated by the ortho, meta or para prefix. A few disubstituted benzenes have singular names given to specific isomers (e.g. salicylic acid & resorcinol). Finally, if there are three or more substituent groups, the ring is numbered in such a way as to assign the substituents the lowest possible numbers, as illustrated by the last row of examples. The substituents are listed alphabetically in the final name. If the substitution is symmetrical (third example from the left) the numbering corresponds to the alphabetical order.
All the best.
AKASH GOYAL
AskiitiansExpert-IIT Delhi
Please feel free to post as many doubts on our discussion forum as you can. We are all IITians and here to help you in your IIT JEE preparation.
Win exciting gifts by answering the questions on Discussion Forum. So help discuss any query on askiitians forum and become an Elite Expert League askiitian.
Now you score 5+15 POINTS by uploading your Pic and Downloading the Askiitians Toolbar respectively : Click here to download the toolbar..



