Crystal Lattices and Unit Cells
Table of contents |
Crystal Lattice
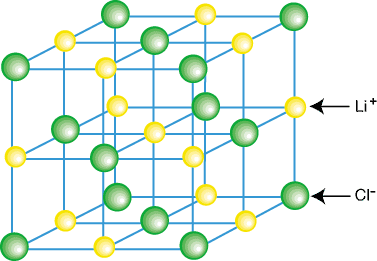
Crystal Lattice is a three-dimensional representation of atoms and molecules arranged in a specific order/pattern. In other words, a crystal lattice can be defined as a geometrical arrangement of constituent particles of matter (atoms, ions or molecules) as points in space. There are total 14 possible three-dimensional lattices. Crystal lattices are also known by Bravais Lattices, named after the scientist Auguste Bravais.
Image 1: Example of a crystal lattice
Characteristics of Crystal Lattices
The following characteristics are depicted by Bravais lattices:
-
Each point in a lattice represents a lattice site or we can say lattice point
-
Each point denotes a particular type of constituent particles of matter be it an atom, molecule or an ion
-
By joining the lattices points inside the lattice we can define geometry of the lattice
Unit Cell
Image 2: A unit cell is the shortest portion of a lattice
The smallest possible portion or part of the crystal lattice which repeats itself in different directions of the lattice is called the unit cell. Many unit cells combine to geometrically form the crystal lattice.
Characteristics of Unit Cell
The following characteristics define a unit cell:
-
A unit cell has three edges a, b and c and three angles α, β and γ between the respective edges
-
The angle between edge b and c is α, a and c is β and that of between a and b is γ
Unit cells are of two types namely:
-
Primitive Unit Cells
-
Centred Unit Cells
Image 3: Types of Unit Cells
Primitive Unit Cells
The unit cell in which the constituent particles (atoms, ions or molecules) are located only on the corners of the lattice is called A Primitive Unit Cell.
Centred Unit Cells
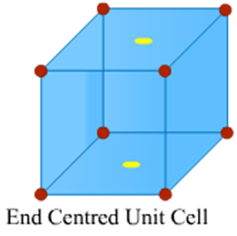
Image 4: End Centred Cell
The unit cell in which the constituent particles (atoms, ions or molecules) are located on the corners, as well as other positions of the lattice, is known as Centred Unit Cells. A centred unit cell is further divided into three types:
-
Body Centred Unit Cells
-
Face Centred Unit Cells
-
End Centred Unit Cells
The unit cell which contains one constituent particle (atom, molecule or ion) at its body centre and other constituent particles are located on the corners is called Body Centred Unit Cells.
Face Centred Unit Cells
The unit cell which contains constituent particles (atoms, molecules or ions) on each face of the unit cell and other constituent particles on the corners is called the Face Centred Unit Cell.
End Centred Unit Cells
In an end centred unit cell, one constituent particle (atom, molecule or ion) is present at the centre of opposite faces besides the ones located on the corners.
Formation of Lattice
Image 5: Types of unit cells which form crystal lattices
Formation of unit cells takes place in seven forms, namely:
-
Cubic Lattice
-
Tetragonal Lattice
-
Orthorhombic Lattice
-
Monoclinic Lattice
-
Hexagonal Lattice
-
Rhombohedral Lattice
-
Triclinic Lattice
Cubic Lattice: Cubic lattice is formed into three possible geometries of unit cells: primitive, body-centred and face centred unit cells. In a cubic lattice, all the edges are equal and the angle between their faces is 90° that is, mutually perpendicular.
Tetragonal Lattice: The formation of tetragonal lattice takes place in two geometries of unit cells: primitive and body centred unit cells. In a tetragonal lattice, only one edge has different length and angle between respective edges is 90° that is, mutually perpendicular
Orthorhombic Lattice: There are four types of orthorhombic lattice mainly: primitive, end-centred, body-centred and face centred. In orthorhombic lattice, the edge lengths are unequal in nature and the angle between respective edges is 90° that is, mutually perpendicular
Monoclinic Lattice: Monoclinic lattice is formed from two types of unit cells namely: primitive and end centred. Monoclinic lattice has unequal sides and two angles between the faces of the lattice are 90°.
Hexagonal Lattice: Hexagonal lattice is formed from only one type of unit cell that is, primitive. In hexagonal lattice, only one side and two angles are 90° and one angle is 120°.
Rhombohedral Lattice: Rhombohedral Lattice is also formed from one type of unit cell that is, primitive. In Rhombohedral Lattice all the sides are equal and two angles between the faces of the rhombohedral lattice are less than 90°.
Triclinic Lattice: The formation of triclinic lattice also takes place from one type of unit cell that is, primitive. In triclinic lattice all the sides are unequal and none of the angles between the faces of the triclinic lattice are 90°.
The table given below can be used to summarize types of lattice formation.
|
Lattice |
Types |
Edge Length |
Angles between faces |
Examples |
|
Cubic |
Primitive, Body-centred, Face-centred |
a = b = c |
α = β = γ = 90° |
NaCl, Copper and ZnS |
|
Tetragonal |
Primitive, Body-centred |
a = b ≠ c |
α = β = γ = 90° |
White tin, SnO2, TiO2 and CaSO4 |
|
Orthorhombic |
Primitive, Body-centred, Face-centred , End-centred |
a ≠ b ≠ c |
α = β = γ = 90° |
Rhombic Sulphur, BaSO4 and KNO3 |
|
Hexagonal |
Primitive |
a = b ≠ c |
α = β = 90° and γ = 120° |
Graphite, ZnO and CdS |
|
Rhombohedral |
Primitive |
a = b = c |
α = β = γ ≠ 90° |
CaCO3 ( Calcite) and HgS (cinnabar) |
|
Monoclinic |
Primitive, End-centred |
a ≠ b ≠ c |
α = γ = 90° β ≠ 90° |
Sulphur |
|
Triclinic |
Primitive |
a ≠ b ≠ c |
α ≠ β ≠ γ ≠ 900 |
H3PO3 , CuSO4.5 H2O |
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free