Basicity of Amine
Aliphatic Bases
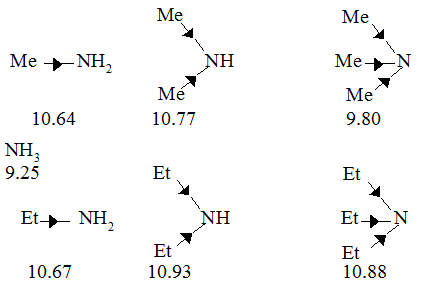
 As increasing strength in nitrogenous bases is related to the readiness with which they are prepared to take up protons, and therefore, to the availability of the unshared electron pair on nitrogen, we might expect to see an increase in basic strength on going : NH3 ® RNH2 ® R2NH® R3N, due to the increasing inductive effect of successive alkyl groups making the nitrogen atom more negative. An actual series of amines was found to have related values as follows, however :
As increasing strength in nitrogenous bases is related to the readiness with which they are prepared to take up protons, and therefore, to the availability of the unshared electron pair on nitrogen, we might expect to see an increase in basic strength on going : NH3 ® RNH2 ® R2NH® R3N, due to the increasing inductive effect of successive alkyl groups making the nitrogen atom more negative. An actual series of amines was found to have related values as follows, however :
It will be seen that the introduction of an alkyl group into ammonia increases the basic strength markedly as expected. The introduction of a second alkyl group further increases the basic strength, but the net effect of introducing the second alkyl group is very much less marked than with the first. The introduction of a third alkyl group to yield a tertiary amine, however, actually decreases the basic strength in both the series quoted. This is due to the fact that the basic strength of an amine in water is determined not only by electron - availability on the nitrogen atom, but also by the extent to which the cation, formed by uptake of a proton, can undergo solvation, and so become stabilized. The more hydrogen atoms attached to nitrogen in the cation, the greater the possibilities of powerful solvation via hydrogen bonding between these and water :
Thus on going along the series, NH3 ® RNH2 ® R2NH ® R3N, the inductive effect will tend to increase the basicity, but progressively less stabilisation of the cation by hydration will occur which will tend to decrease the basicity. The net replacing effect of introducing successive alkyl groups thus becomes progressively smaller, and an actual changeover takes place on going from a secondary to a tertiary amine. If this is the real explanation, no such changeover should be observed if measurements of basicity are made in a solvent in which hydrogen - bonding cannot take place; it has, indeed, been found that in chlorobenzene the order of basicity of the butylamines is
BuNH2 < Bu2NH < Bu3N
Tetralkylammonium salts, e.g. R4NÅ I-, are known, on treatment with moist silver oxide, AgOH, to yield basic solution comparable in strength with the mineral alkalis. This is readily understandable for the base so obtained, R4N+ –OH, is bound to be completely ionised as there is no possibility, as with tertiary amines, etc.,
R3NH++ -OH → R3N: + H2O
of reverting to an unionised form.
 The effect of introducing electron withdrawing groups, e.g. Cl, NO2, close to a basic center is to decrease the basicity, due to their electron withdrawing inductive effect. Thus the amine is found to be virtually non - basic, due to the three powerfully electron withdrawing CF3 groups. This can well be explained on the basis of more ‘s’ character on lone pair of N.
The effect of introducing electron withdrawing groups, e.g. Cl, NO2, close to a basic center is to decrease the basicity, due to their electron withdrawing inductive effect. Thus the amine is found to be virtually non - basic, due to the three powerfully electron withdrawing CF3 groups. This can well be explained on the basis of more ‘s’ character on lone pair of N.The change is also pronounced with C=O, for not only is the nitrogen atom, with its electron pair, bonded to an electron withdrawing group through an sp2 hybridised carbon atom but an electron withdrawing mesomeric effect can also operate:
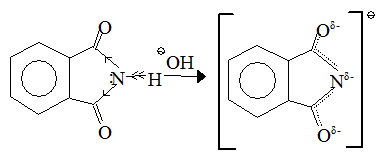
Thus amides are found to be only very weakly basic in water [pKa for ethanamide(acetamide) is » 0.5], and if two C=O groups are present the resultant imides, far from being basic, are often sufficiently acidic to form alkali metal salts, e.g. benzene - 1, 2 - dicarboximide :
Aromatic Bases: (Aromatic Amines)
 ?The exact reverse of the above is seen with aniline, which is a very weak base (pKa = 4.62) compared with ammonia (pKa = 9.25) or cyclohexylamine (pKa = 10.68). In aniline the nitrogen atom is again bonded to a sp2 hybridised carbon atom but, more significantly, the unshared electron pair on nitrogen can interact with the delocalised p orbitals of the nucleus. If aniline is protonated, any such interaction, with resultant stabilisation, in the anilinium cation is prohibited, as the electron pair on N is no longer available
?The exact reverse of the above is seen with aniline, which is a very weak base (pKa = 4.62) compared with ammonia (pKa = 9.25) or cyclohexylamine (pKa = 10.68). In aniline the nitrogen atom is again bonded to a sp2 hybridised carbon atom but, more significantly, the unshared electron pair on nitrogen can interact with the delocalised p orbitals of the nucleus. If aniline is protonated, any such interaction, with resultant stabilisation, in the anilinium cation is prohibited, as the electron pair on N is no longer available  The aniline molecule is thus stabilised with respect to the anilinium cation, and it is therefore ‘energetically unprofitable’ for aniline to take up a proton ; it thus functions as a base with the utmost reluctance (pKa = 4.62, compared with cyclohexylamine,
The aniline molecule is thus stabilised with respect to the anilinium cation, and it is therefore ‘energetically unprofitable’ for aniline to take up a proton ; it thus functions as a base with the utmost reluctance (pKa = 4.62, compared with cyclohexylamine,
pKa = 10.68). The base weakening effect is naturally more pronounced when further phenyl groups are introduced on the nitrogen atom ; thus diphenylamine, Ph2NH, is an extremely weak base (pKa = 0.8), while triphenylamine, Ph3N, is by ordinary standards not basic at all. Introduction of alkyl, e.g. Me, groups, on to the nitrogen atom of aniline results in small increase in pKa :
C6H5NH2 C6H5NHMe C6H5NMe2 MeC6H4NH2
4.62 4.84 5.15 o-4.38
m-4.67
p-5.10
Unlike on such introduction in aliphatic amines this small increase is progressive : suggesting that cation stabilisation through hydrogen - bonded solvation, responsible for the irregular behavior of aliphatic amines, here has less influence on the overall effect. The major determinant of basic strength in alkyl-substituted anilines remains mesomeric stabilisation of the aniline molecule with respect to the cation ; borne out by the irregular effect of introducing Me groups into the o-, m- and p-positions in aniline.
A group with a more powerful (electron - withdrawing) inductive effect, e.g. NO2 is found to have rather more influence. Electron withdrawal is intensified when the nitro group is in the o- or p-position, for the interaction of the unshared pair of the amino nitrogen with the delocalised p orbital sytsem of the benzene nucleus is then enhanced. The neutral molecule is thus stabilised even further with respect to the cation, resulting in further weakening as a base. Thus the nitro - anilines are found to have related pKa values :
The extra base - weakening effect, when the substituent is in the o-position, is due in part to the short distance, over which its inductive effect is operating, and also to direct interaction, both steric and by hydrogen bonding, with the NH2 group. o-Nitroaniline is such a weak base that its salts are largely hydrolysed in aqueous solution, while 2, 4 - dinitroaniline is insoluble in aqueous acids, and 2, 4, 6 - trinitroaniline resembles an amide; it is indeed called picramide and readily undergoes hydrolysis to picric acid (2, 4, 6 - trinitrophenol).
With substituents such as OH and OMe that have unshared electron pairs, an electron - donating, i.e. base strengthening, mesomeric effect can be exerted from the o- and p-, but not from the m-position, with the result that the p-substituted aniline is a stronger base than the corresponding m-compound. The m-compound is a weaker base than aniline itself, due to the electron - withdrawing inductive effect exerted by the oxygen atom in each case. As so often, the effect of the o - substituent remains somewhat anomalous, due to the interaction with the NH2 group by both steric and polar effects. The substituted anilines are found to have related pKa values.
You can also refer JEE Organic Chemistry Syllabus, & Reference books of Organic Chemistry
To read more, Buy study materials of Amines comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.