Amino Acids
Table of Content |
Amino Acids are molecules, which contain two functional groups, one is carboxylic group and another is amino group. Amino acids are derivatives of carboxylic acids in which one hydrogen atom of carbon chain is substituted by Amino group. Amino group may be at alpha, beta or gama position with respect to carboxylic group
|
Formula of amino acid |
Name of Amino Acids |
|
H2N-CH2 - COOH |
Amino acetic acid, or Glycine |
|
CH3 - CH (NH2) - COOH |
|
|
H2N - CH2 - CH2 -COOH |
|
|
H2N - CH2 - (CH2)2 - COOH |
|
Some amino acids contain a second carboxyl group or a potential carboxyl group in the form of carboxamide: these are called acidic amino acid some contain a second basic group which may be an amino group these are called basic amino acids.
-
Physical Properties of Amino Acids
?Although the amino acids are commonly shown as containing an amino group and a carboxyl group, certain properties are not consistent with this structure. In contrast to amines and carboxylic acids, the amino acids are nonvolatile solids, which melt at fairly high temperatures.They are insoluble in organic solvents [i.e. non polar solvents] and are highly soluble in water.Their aqueous solution is neutral.Their aqueous solutions behave like solutions of substances of high dipole moment. Acidity and basicity constants are ridiculously low for - COOH and – NH2 groups In the physical properties melting points, solubility, and high dipole moment are just what would be expected of such a salt. The acid base properties also become understandable when it is realized that the measured Ka actually refers to the acidity of an ammonium ion, RNH3+

and Kb actually refers to the basicity of a carboxylate ion, RCOO–

When the solution of an amino acid is made alkaline, the dipolar ion(I) is converted to the anion (II); the stronger base, hydroxide ion, removes a proton from the ammonium ion and displaces the weaker base, the amine
+H3N ¾ CHRCOO– + OH–  H2N CHRCOO– + H2O
H2N CHRCOO– + H2O
(I) (II)
Stronger Stronger Weaker Weaker
acid base base acid
When the solution of an amino acid is made acidic; the dipolar ion I is converted into the cation (III); the stronger acid H3O+, gives up a proton to the carboxylate ion, and displaces the weaker carboxylic acid.
+H3N CHRCOO– + H3O+  +H3N CHRCOOH + H2O
+H3N CHRCOOH + H2O
(I) (III)
Stronger Stronger Weaker Weaker
base acid acid base
In summary, the acidic group of a simple amino acid like glycine is –NH3+.not –COOH, and basic group is –COO- not –NH2.
-
Classification of Amino Acid
Amino acid with non – polar side chain

Acidic Amino Acid: These amino acids contain a second carboxyl group or a potential carboxyl group in the form of carboxamide.
Basic Amino Acids: These contain a second basic group which may be an amino group.

-
Essential & Non-Essential Amino Acids
Those amino acids which must be supplied to our diet as are not synthesized in body are known as essential amino acids. Some of them are
Valine, Leucine, Isoelucine, Phenylalanine, Arganine, Threonine , Tryptophan, Methionine, Lysine, Arginine, Histadine
Note: Histidine and arginine are essential i.e. can be syntrhesized but not in quantities sufficient to permit normal growth.
Those amino acids which are synthesized in body are non-essential amino acids. Some of them are.
Glycine, Alanine, Tyrosine, Serine, Cystine, Proline, Hydroxyprocine , Cysteine, Aspartic acid, Glutonic acid
-
Zwiter Ion
Amino acids contain both acidic carboxyl group -(COOH) and basic amino group in the same molecules. In aqueous solution, the acidic carboxyl group can lose a proton and basic amino group can gain a proton in a kind of internal acid – base reaction. The product of this internal reaction is called a Dipolar or a Zwitter ion. The Zwitter ion is dipolar, changed but overall electrically neutral and contain both a positive and negative charge. Amino acid in the dipolar ion form are amphoteric in nature. Depending upon the pH of the solution, the amino acid can donate or accept proton.
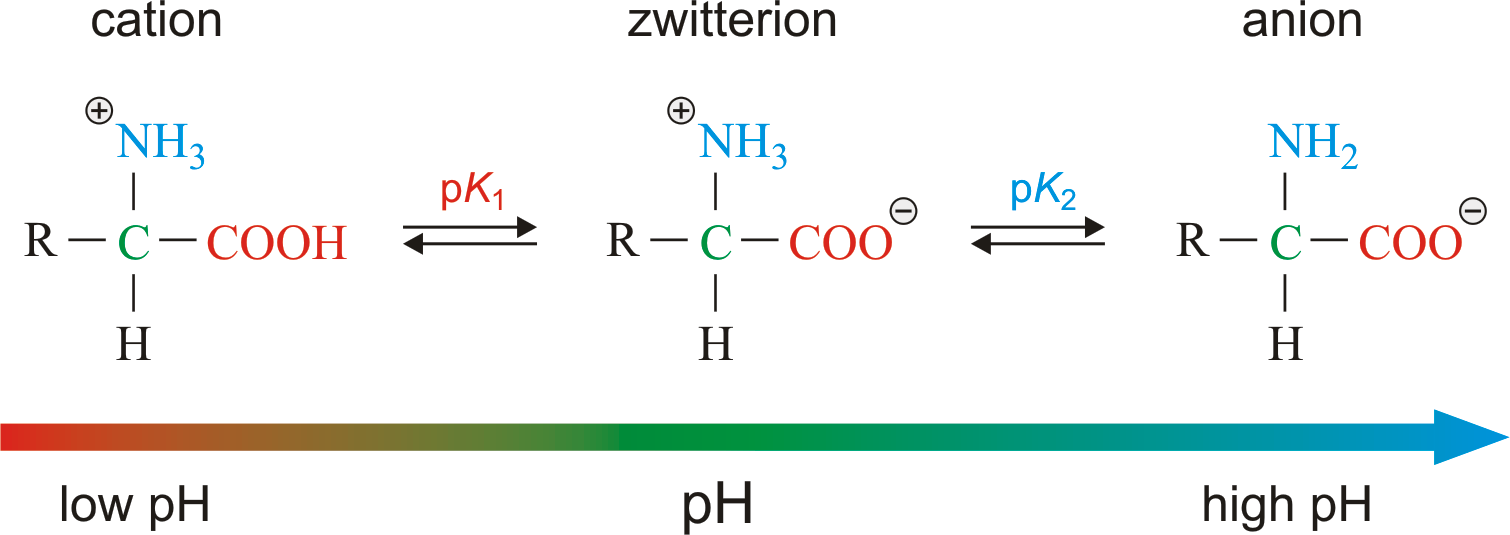
-
Iso Electric Point of Amino Acids?
When ionized form of amino acid is placed in an electric field it will migrate towards the opposite electrode. Depending upon the pH of the medium following three thing may happen.
- In acidic medium, the cation move towards cathode.
- In basic medium, the anion move towards anode.
- The Zwitter ion does not move towards any of the electrodes.
At a certain pH (i.e. H+ concentration), the amino acid molecules show no tendency to migrate towards any of the electrodes and exists as a neutral dipolar ion, when placed in electric field is known as isoelectric point. All amino acids do not have the same isoelectric point & it depends upon the nature of R – linked to α- carbon atom.
|
Amino Acids |
Isoelectric point |
|
Neutral amino acids |
(pH 5.5 to 6.3) |
|
Glycine |
5.7 |
|
Alanine |
6.1 |
|
Valine |
6.0 |
|
Serine |
5.7 |
|
Threonine |
5.6 |
|
Acidic amino acids |
(pH » 3) |
|
Aspartic acid |
2.8 |
|
Glutamic acid |
3.2 |
|
Basic amino acids |
(pH » 10) |
|
Lysine |
9.7 |
|
Arginine |
10.8
|
Amino acids have minimum aqueous solubility at isoelectric point.

-
Synthesis of α - Amino Acids
-
Protein can be hydrolyzed by refluxing with dilute hydrochloric acid to give a mixture of α - amino acids. The resulting mixture can be separated by fractional crystallization.
-
Fractional distillation of their ester followed by hydrolysis (Fischer’s method)
-
Selective precipitation as salt with phosphotungstic and picric acid.
-
Distribution of amino acid between n – butanol saturated with water (Dakin’s method).
-
Column, paper and gas chromatography.
-
Electrophoresis.
By amination of α- halo acid

By Strecker Synthesis
(i)
(ii)

Note: Generally the aldehyde is treated with a mixture of ammonium chloride and potassium cyanide in aqueous solution

-
Chemical Properties of Amino Acids
Amino acids show the following characteristic reactions.
1.Reaction of the carboxyl group.
2.Reaction of the amino group.
3.Reaction involving both the carboxyl and the amino group.
Reaction of the carboxyl group
Reaction with base

-
Esterification

Note: HCl first converts the dipolar ion into an acid which is subsequently esterified.
Decarboxylation

Reduction

Reaction with strong acid

Acetylation


Reaction with Nitrous acid

Note:
-
This reaction forms the basis of the “van slyke method” for the estimation of amino acids.
-
The nitrogen is evolved (one half comes from the amino acid) quantitatively and its volume measured.
Reaction with Nitrosyl halide
-
This reaction forms the basis of the “van slyke method” for the estimation of amino acids.
-
The nitrogen is evolved (one half comes from the amino acid) quantitatively and its volume measured.
Reaction with Nitrosyl halide

Reaction with 2, 4 – Dintrofluorobenzene (DNFB)

Reaction involving both the carboxyl & the amino group
Effect of heat
α - amino acids undergo dehydration on heating (200°C) to give diketo piperazines

You can also Refer to
JEE Organic Chemistry Syllabus
Reference books of Organic Chemistry
To read more, Buy study materials of Biomolecules comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.


 - Amino propionic acid or Alanine
- Amino propionic acid or Alanine - Amino propionic acid
- Amino propionic acid - Amino butyric acid
- Amino butyric acid