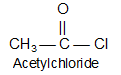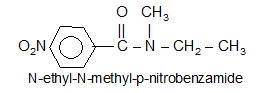IIT JEE Carboxylic Acids and Its Derivatives | JEE Derivatives of Carboxylic Acid
Table of Content |
Introduction to Carboxylic Acids and Its Derivatives
Of the organic compounds that show appreciable acidity, by far the most important are the carboxylic acids.
 These compounds contain the carboxyl group attached to hydrogen (HCOOH) an alkyl group (RCOOH), or an aryl group (ArCOOH). These are also named as fatty acids because of some higher members particularly palmitic and stearic acids, occur in natural fats. The general formula of the carboxylic acids is CnH2nO2.
These compounds contain the carboxyl group attached to hydrogen (HCOOH) an alkyl group (RCOOH), or an aryl group (ArCOOH). These are also named as fatty acids because of some higher members particularly palmitic and stearic acids, occur in natural fats. The general formula of the carboxylic acids is CnH2nO2.
Only the hydrogen atom of the carboxyl group is replaceable by a metal, therefore the fatty acids are mono-basic.
Carboxylic acids are characterized by the presence of carboxyl group. The –COOH group which itself is made up of a carbonyl group (C=O) and a hydroxyl group (¾OH) is called carboxyl group (carb from carbonyl and oxyl from hydroxyl)
Carboxylic acids may be aliphatic or aromatic
Comparison of resonating structures of carboxylic group and carbonyl group. Carbonyl group has two resonance structures (I and II)
However, for a carboxyl group, three resonance structures (A, B and C) can be written.
In both structures (A) and (C), the C – atom and the two O – atoms have eight electrons in their respective valence shells while in structure (B), C – atom has only six electrons. Therefore, structure (B) is less stable than structure (C), in other words the two important resonance structures of carboxyl group are structures (A) and (C). In both these structures, carboxyl carbon is electrically neutral. However in case of aldehydes and ketones, only one structure i.e. I is electrically neutral. As a result, the carboxyl carbon of the resonance hybrid is less positive and hence less electrophilic than the carbonyl carbon of aldehydes and ketones. However, it may be noted that like carbonyl group, carboxyl group is also polar due to resonance structures (B) and (C)
Carboxylic Acids and Its Derivativs is one of the most scoring and important unit in the entire preparation of IIT JEE, and other engineering entrance examinations. It is of no doubt that along with amides, alkenes etc it is one of the most scoring of all topics in Organic Chemistry.
Carboxylic Acids and Its Derivativs includes topics
which are very important from the point of view that these are prerequisite to Organic chemistry Sections.
Nomenclature of Carboxylic Acids
The aliphatic carboxylic acids are commonly known by their initial names, which have been derived from the source of the particular acid.
Examples:
Carboxylic Acid |
Common Name |
| HCOOH | Formic acid [Latin: Fermica = ant] |
| CH3COOH | Acetic acid [Latin: acetum = vinegar] |
| CH3–CH2–COOH | Propionic acid |
| CH3(CH2)2COOH | Butyric acid |
| CH3(CH2)3COOH | Valeric acid |
| CH3(CH2)14COOH | Palmitic acid |
| CH3(CH2)16COOH | Stearic acid |
Another system of nomenclature, except Formic acid considers acids as acid derivatives of acetic acid
Example:
CH3 – CH2 – COOH Methyl acetic acid
(CH3)3C – COOH Trimethyl acetic acid
According to the IUPAC system of nomenclature, the suffix of the monocarboxylic acid is ‘oic acid’, which is added to the name of the alkane corresponding to the longest carbon chain containing the carboxyl group, e.g.
HCOOH methanoic acid
CH3 – CH2 – CH2 – COOH butanoic acid
The positions of side-chains (or substituents) are indicated by numbers, the carboxyl group always being given number I.
Naming of Acyl Groups; Acid Chlorides and Acid Anhydrides
The group obtained from a carboxylic acid by the removal of the hydroxyl portion is known as an acyl group. The name of an acyl group is created by changing the 'ic' at the end of the name of the carboxylic acid to 'yl', examples:
Acid chlorides are named systematically as acyl chlorides.
An acid anhydride is named by substituting anhydride for acid in the name of the acid from which it is derived.
Naming Salts and Esters
The name of the cation (in the case of a salt) or the name of the organic group attached to the oxygen or the carboxyl group (in the case of an ester) preceeds the name of the acid. The 'ic acid’ part of the name of the acid is converted to 'ate'
Name of Amides and Imides
The names of amides are formed by replacing –oic acid (or –ic acid for common names) by amide or –carboxylic acid by carboxamide.
If the nitrogen atom of the amide has any alkyl group as substituent, the name of the amide is prefixed by the capital letter N; to indicate substitution on nitrogen, followed by the name(s) of alkyl group(s).
If the substituent on the nitrogen atom of an amide is a phenyl group, the ending for the name of the carboxylic acid is changed to anilide
some dicarboxylic acids form cylic amides in which two acyl groups are bonded to the nitrogen atom. The suffix imide is given to such compounds.
Physical Properties of Carboxylic Acids
The molecules of carboxylic acids are polar and exhibit hydrogen bonding. The first four are miscible with water. The higher acids are virtually insoluble. The simplest aromatic acid, benzoic acid, contains too many carbon atoms to show appreciable solubility in water.
Carboxylic acids are soluble in less polar solvents like ether, alcohol, benzene etc.
Carboxylic acids have higher boiling points than alcohols. These very high boiling points are due to the fact that a pair of carboxylic acid molecules are held together not by one but by two hydrogen bonds and exist as dimer. The first three fatty acids are colourless pungent smelling liquids. A study of nitrated spectra of formic acid in the liquid and solid states has provided evidence that this acid, unlike most of the other carboxylic acids, is not dimeric in these states, but is associated as a polymer.
Acidity of Carboxylic Acids
The acidity of a carboxylic acid is due to the resonance stabilization of its anion
Because of the resonance, both the carbon oxygen bond in the carboxylate anion have identical bond length. In the carboxylic acid, these bond lengths are no longer identical.
The acidity of carboxylic acid depends very much on the substituent attached to – COOH group. Since acidity is due to the resonance stabilization of anion, substituent causing stabilization of anion increases acidity whereas substituent causing destabilization of anion decrease acidity. For example, electron withdrawing group disperses the negative charge of the anion and hence makes it more stable causing increase in the acidity of the corresponding acid, on the other hand, electron-releasing group increases the negative charge on the anion and hence makes it less stable causing the decrease in the acidity. In the light of this, the following are the orders of a few substituted carboxylic acids.
a) Increase in the number of Halogen atoms on a-position increases the acidity, eg.
Cl3CCOOH > Cl2CHCOOH > ClCH2COOH > CH3COOH
b) Increase in the distance of Halogen from COOH decreases the acidity e.g.
CH3 – CH2 – CH – COOH > CH3 – CH – CH2 – COOH > CH2 – CH2 – CH2 – COOH
| | |
Cl Cl Cl
This is due to the fact that inductive effect decreases with increasing distance.
c) Increase in the electro negativity of halogen increases the acidity.
FCH2COOH > BrCH2COOH > ICH2COOH
Related Resources
JEE Organic Chemistry Syllabus
Organic Chemistry Revision Notes
Reference books of Organic Chemistry
To read more, Buy study materials of Aldehydes, Ketones & Carboxylic Acids comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free