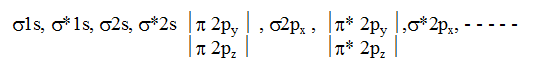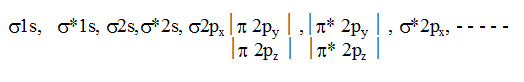IIT JEE Molecular Orbital Theory | JEE Chemical Bonding
Table of Content |
 Introduction to Molecular Orbital Theory
Introduction to Molecular Orbital Theory
One of the most important theories developed is the wave-particle, duality of particles. Electrons can be considered as particles and waves also. Based on this, it can be concluded that electrons behaving as waves can interact with each other and the process is called interference.
As in waves, two types of interferences are possible: (1) constructive and (2) Destructive.
In molecules the atomic orbitals of all the atoms are assumed to interfere with each other in the form of waves and depending on the nature of interferences, two molecular orbitals result. The one which results from constructive interference is called bonding molecular orbital and the one which results from destructive interference is called anti-bonding molecular orbital. Obviously anti-bonding MO is of higher energy than Bonding MO.
In Molecular Orbital Theory (MOT) the atoms in a molecule are supposed to loose their individual control over the electrons. The nuclei of the bonded atoms are considered to be present at equilibrium inter-nuclear positions. The orbitals where the probability of finding the electrons is maximum are multicentred orbitals called molecular orbitals extending over two or more nuclei.
In MOT the atomic orbitals loose their identity and the total number of electrons present are placed in Mo’s according to increasing energy sequence (Auf Bau Principle) with due reference to Pauli’s Exclusion Principle and Hund’s Rule of Maximum Multiplicity.
As mentioned above, when a pair of atomic orbitals combine they give rise to a pair of molecular orbitals, the bonding and the anti-bonding. The number of molecular orbitals produced must always be equal to the number of atomic orbitals involved. Electron density is increased for the bonding MO’s in the inter-nuclear region but decreased for the anti-bonding MO’s, Shielding of the nuclei by increased electron density in bonding MO’s reduces inter nuclei repulsion and thus stabilizes the molecule whereas lower electron density even as compared to the individual atom in anti-bonding MO’s increases the repulsion and destabilizes the system.
Refer to the following video for molecular orbital theory
In simple homonuclear diatomic molecules the order of MO's based on increasing energy is :
For molecules including O2 and above, the order is
This order is true except B2, C2 & N2. If the molecule contains unpaired electrons in MO’s it will be paramagnetic but if all the electrons are paired up then the molecule will be diamagnetic.
Bond Order
Bond order is a number which indicates the no. of bonds a molecule possesses and the stability of the molecule in comparison to another. An integral value implies that so many bonds exist in the molecule. Anything fractional indicates that the bond is intermediate.
Bond-order = 1/2 (no. of bonding electrons - No. of antibonding electrons).
Application of MOT to Homonuclear Diatomic Molecules
H2+ molecule ion. Total no. of electrons = 1
-
Arrangement : s 1s1
-
An unpaired electron always indicates that the molecule is paramagnetic.
H2 molecule, Total no. of electrons = 2
-
Arrangement : s 1s2
-
Paired electrons , so diamagnetic.
-
BO = 1/2 (2-0) = 1
-
Therefore No. of bonds = 1
He2+ molecule ion, Total no. of electrons = 3,
-
Arrangement : s1s2, s* 1s1
-
Unpaired electron, so paramagnetic
-
BO = 1/2 (2-1) = 1/2
-
Bond existing by virtue of a single electron.
He2 molecule. Total No. of electrons = 4,
-
Arrangement : s1s2, s* 1s2
-
Diamagnetic BO = 0
-
Molecule does not exist.
Example: Which diatomic molecule of second period besides O2 should be paramagnetic?
Solution:
![]()
As, paramagnetism arises due to unpaired electron. Therefore B2is paramagnetic molecule.
M.O. of Some Diatomic Heteronuclei Molecules
The molecular orbitals of heteronuclei diatomic molecules should differ from those of homonuclei species because of unequal contribution from the participating atomic orbitals. Let’s take the example of CO.
The M.O. energy level diagram for CO should be similar to that of the isoelectronic molecule N2. But C & O differ much in electronegativity and so will their corresponding atomic orbitals. But the actual MO for this species is very much complicated since it involves a hybridisation approach between the orbital of oxygen and carbon.
HCl Molecule: Combination between the hydrogen 1s A.O’s. and the chlorine 1s, 2s, 2p & 3s orbitals can be ruled out because their energies are too low. The combination of H 1s1 and 3p1x gives both bonding and anti-bonding orbitals, and the 2 electrons occupy the bonding M.O. leaving the anti-bonding MO empty.
NO Molecule: The M.O. of NO is also quite complicated due to energy difference of the atomic orbitals of N and O.
As the M.O.’s of the heteronuclei species are quite complicated, so we should concentrate in knowing the bond order and the magnetic behaviour.
Molecules/Ions |
Total No. of Electrons |
Magnetic Behaviour |
| CO | 14 | Diamagnetic |
| NO | 15 | Paramagnetic |
| NO+ | 14 | Diamagnetic |
| NO– | 16 | Diamagnetic |
| CN | 13 | Paramagnetic |
| CN– | 14 | Diamagnetic |
Inert Pair Effect
Heavier p-block and d-block elements show two oxidation states. One is equal to group number and second is group number minus two. For example Pb(5s25p2) shows two OS, +II and +IV. Here +II is more stable than +IV which arises after loss of all four valence electrons. Reason given for more stability of +II O.S. that 5s2 electrons are reluctant to participate in chemical bonding because bond energy released after the bond formation is less than that required to unpair these electrons (lead forms a weak covalent bond because of greater bond length).
Example: Why does PbI4 not exist?
Solution: Pb(+IV) is less table than Pb(+II) due to inert pair effect and therefore Pb(+IV) is reduced to Pb(+II) by I– which changes to I2(I– is a good reducing agent)
Related Resorces
Reference books of Physical Chemistry
To read more, Buy study materials of Chemical Bonding comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free