Atmospheric Pollution
Table of Content |
What is Atmospheric Pollution?
It is defined as the introduction of harmful materials into the atmosphere is known as Atmospheric Pollution.
Fig.1. Definition of Air Pollution
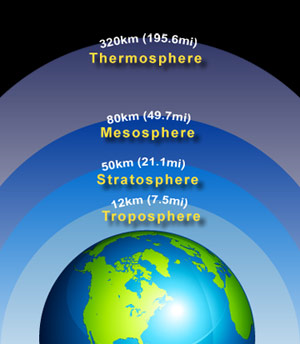
Atmosphere has different layers or regions in which it is divided:
-
Troposphere is the lowest region where living organisms inhabit.
-
Stratosphere is the layer above troposphere where ozone is present.
-
Mesosphere is where the temperature is low. The air is thin to breathe.
-
Thermosphere is the layer where rays from the atmosphere are first absorbed such as X-rays, UV-rays etc.
What is the Definition of Air Pollutants?
Air pollutants can be gases, particulate matter, heavy metals, or any solid particles that pollutes the air.
Causes of Air Pollution
Tropospheric Pollution:
It occurs when some solid or gaseous particles are suspended in air.
Pollutants are divided into gaseous air pollutants and particulate pollutants.
Gaseous Air Pollutants:
-
Oxides of Sulphur are formed when fossil fuels are burnt. Burning of fossil fuels form Sulphur dioxide. Sulphur dioxide is harmful for plants and animals and causes, respiratory disorders. It also causes irritation in eyes such as tears and redness. In plants, it causes flower buds to fall from the plant. The reactions are follows:
2SO2 (g) +O2 (g) → 2SO3(g)
SO2 (g) +O3 (g) → SO3(g) + O2 (g)
SO2(g) + H2O2(l) → H2SO4(aq)
The oxides of Sulphur are potential agents that causes acid rain.
-
Oxides of Nitrogen are formed when lightning strikes the oxygen at higher altitude. During burning of fossil fuels at high temperature, oxygen and nitrogen combines to form nitrogen dioxide and nitric oxide.
2NO +O2 → 2NO2
Nitric oxide than reacts further with oxygen to form nitrogen dioxide.
2NO +O2 → 2NO2
NO formed also reacts with ozone to from nitrogen dioxide and oxygen. High concentration of nitrogen dioxide causes damage to plant leaves. It also causes respiratory problems.
-
Hydrocarbons are composed of hydrogen and carbon. Incomplete combustion of fossil fuels used in automobiles forms hydrocarbons. They are carcinogenic. They cause ageing in plants, shedding of flowers and leaves etc.
-
Oxides of Carbon includes carbon monoxide and carbon-dioxide. Carbon monoxide is one of the toxic pollutant. Carbon monoxide has a strong affinity to bind with hemoglobin. This reduces the amount of oxygen that bind with the hemoglobin. This forms carboxyhemoglobin which causes reduce availability of oxygen to tissues.
Carbon Dioxide is added to the atmosphere by respiration, burning of fossil fuels, and decomposition of limestone. Released carbon-dioxide is taken in by plants during photosynthesis to balance the carbon-dioxide released in the atmosphere. But as deforestation is increasing, the rate of carbon-dioxide taken in by plants decreases. The effects of increased carbon-dioxide in atmosphere are Global warming and Green-house effect.
Global Warming
Most of the energy released by the sun is retained by the atmosphere which raises the temperature.The rest of the heat radiates back to the atmosphere. Gases trap this heat, for example, carbon dioxide, methane, ozone, Chlorofluorocarbon Compounds (CFCs) and water vapor present in the atmosphere. This raises the temperature of the atmosphere; this is known as Global Warming.
Green-House Effect occurs when flowers, vegetables and fruits are grown in glass covered areas. Glass has the capability to capture energy from the sun and prevents the heat to reflect in the atmosphere. This increases the temperature in the green house. This is known as Green-House Effect. The solar radiations heat up the plants and soil. Green-house gases includes carbon-dioxide, water vapor, methane, nitrous oxide, CFCs, and the ozone.
Particulate Pollutants
It includes minute solid particles or liquid droplets suspended in air. The source of particulate pollutants can be smoke particles from fires, vehicle emissions, ash, dust particles etc. Bacteria, viruses, moulds, algae also act as particulate pollutant.
Classification of non-viable particulate pollutants are as follows:
-
Smoke particulates formed during combustion of organic matter. For example, garbage, burning of fossil fuels, smoke from burning of oil.
-
Dust are particles that are formed due to various sources such as crushing or grinding of solid particles. Sand, saw dust, fly ash, etc. are also important source of particulate matter.
-
Mists are small water droplets that decreases the visibility in the atmosphere. For example, Mist from herbicide and insecticides.
-
Fumes are another non-viable particulate pollutants that are formed due to condensation of vapors during distillation, boiling etc.
Smog
Combination of smoke and fog forms smog. There are two types of smog:
-
Classical smog is a mixture of smoke, Sulphur dioxide and fog. It occurs in cool and humid environment.
-
Photochemical smog is a combination of formaldehyde, acrolein, nitric oxide, ozone, and PAN. It occurs in warm and sunny environment.
Formation of Photochemical Smog
During the burning of fossil fuels, a large variety of pollutants are released into the environment. The main pollutants released during burning includes hydrocarbons and nitric oxide. When the concentration of these pollutants increases to a certain level, a chain of reaction occurs with sunlight to form nitrogen dioxide
NO2(g) → NO(g) + O(g)
When nitrogen dioxide absorbs energy, it breaks down into nitric oxide and oxygen atom. Oxygen atom is highly reactive, so it combines with oxygen to form ozone.
O(g) + O2(g) → O3(g)
Ozone combines with nitric oxide to form nitrogen dioxide and oxygen.
NO(g) + O3(g) → NO2(g) + O2(g)
Ozone is toxic and is a strong reducing agent which can react with unburnt hydrocarbons to produce 3 products- acrolein, peroxyacetyl nitrate (PAN) and formaldehyde.
Effects of Photochemical Smog
-
Causes eye irritation
-
Headache, chest pain, cough, difficulty in breathing
-
Damage to the plant life
-
Corrosion of stones and metals
Fig. 4. Particulate Pollutants
Stratospheric Pollution
Stratosphere contains ozone layer that protects the earth from harmful ultraviolet rays. Th wavelength of these harmful ultraviolet radiation is 255 nm. These ultraviolet radiations cause skin cancer in humans. Oxygen when absorbs ultraviolet rays, it breaks into oxygen atoms. These oxygen atoms when combine with oxygen, they form ozone.
But now-a-days, there are reports which suggest that ozone layer is getting depleted by certain pollutants. The main pollutant behind the ozone depletion is Chlorofluorocarbon (CFCs) known as Freon. CFCs are used in refrigerators. These CFCs in atmosphere absorbs ultraviolet rays and forms chlorine free radical.
CF2Cl2 (g) → Chlorineo (g) + Co F2Cl (g)
The chlorine radical reacts with ozone to form chlorine monoxide and molecular oxygen.
Clo (g) + O3 (g) → ClOo (g) + O2(g)
Chlorine monoxide than reacts with oxygen atom to produce chlorine free radical.
ClOo(g) + Oo (g) → Clo (g) + O2 (g)
These chlorine free radicals formed are continuously used to breakdown the ozone.
Ozone Hole
During 1980s, scientists had reported that there is depletion of ozone in the Antarctica region, this is known as ozone hole. During summer time, nitrogen dioxide and methane combines with chlorine monoxide and chlorine atoms forming chlorine sinks and thus prevents ozone depletion. But during winter season, polar stratospheric clouds are formed over Antarctica. These are special clouds that provide a surface for chlorine nitrate formation.
These clouds then get hydrolyzed to form hypochlorous acid. Hypochlorous acid the combines with hydrogen chloride to form molecular chlorine.
Fig. 5. Ozone Depletion
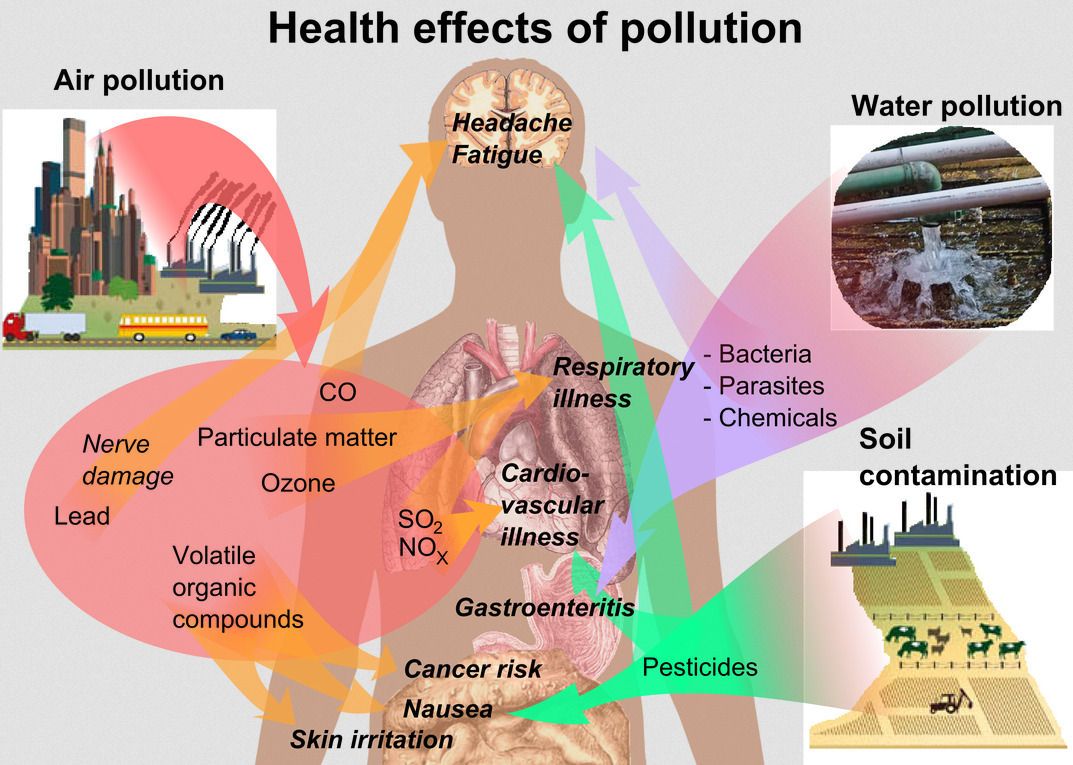
Effects of Air Pollution
-
Acid rain is the most common result of air pollution. Acidification causes damage to both plants and animals.
-
Ozone depletion is another important effect of atmospheric pollution.
-
Photochemical smog is another effect of air pollution.
Fig. 6. Effects of Atmospheric Pollution
Watch this Video for more reference
More Readings