Solved Examples on Hydrocarbons
Question:1
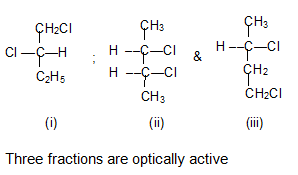
Chlorination of optically active 2–chlorobutane yields a mixture of isomers with the formula C4H8Cl2.
a) How many different isomers would you expect to be produced? What are their structures?
b) Which of these fractions would be optically active?
Solution:
a)

b) The compounds which are having chiral centers (asymmetric carbon) will be optically active
Question 2: Suggest a mechanism for the dehydration of CH3CHOHCH3 that proceeds through a carbonium ion intermediate. Assign a catalytic role to the acid and keep in mind that the O in ROH is a basic site like O in H2O.
Solution:

H2SO4 acts as an acid in first step and base in third step. Since it is regenerated back it is acting as a catalyst.
Question 3: List the possible products, in order of decreasing yield, from the reaction of 3-bromo –2,3 – dimethyl pentane with alcoholic KOH.
Solution:

According to Saytzeff rule, more substituted alkene is more stable
Question 4: Account for the formation of both 3 – bromo –2,2– dimethyl butane and 2–bromo –2,3 dimethyl butane from the reaction of HBr with 2,3–dimethyl –1- butene.
Solution:

The addition of HBr to some alkene gives a mixture of expected alkyl bromide and an isomer formed by rearrangement.
Question 5: Use HC º CH as the only organic reagent to prepare (a) (E) –3– hexene and (b) (Z) –3– hexene
Solution:

Question 6:
Deduce the structural formula of a compound, C10H10
(A), that gives as the only organic compound, 


(B) on oxidative cleavage, C10H10 is the molecular formula of the organic compound
The degree of unsaturation in the compound is = (number of carbon) – (Number of Hydrogen) /2 +1  = (10+1) –
= (10+1) –  = 11 – 5 = 6
= 11 – 5 = 6
This compound contains 6 bonds on oxidative cleavage it is producing only one compound, so all the multiple bonds should be at the terminal atom.

Question 7: 0.37gm of ROH was added to CH3MgI and the gas evolved measured 11.2c at STP. What is the molecular wt of ROH? On dehydration ROH gives an alkene which on ozonolysis gives acetone as one of the products. ROH on oxidation easily gives an acid containing the same number of carbon atoms. Give the structures or ROH and the acid with proper reasoning.
Solution:

Question 8: A chloro compound A showed the following properties.
- decolorises bromine in CCl4
- absorbs hydrogen catalytically
- gives a ppt with ammonical cuprous chloride
- when vapourised 1.49g of A gives 448 ml of vapour at STP. Identify A and write down the equation of the reaction at Step (C).
Solution:
PV = nRT = W/M RT = (1.49 × 0.821 × 273)/(1×448×10-3)
The compound A decolourises bromine and absorbs hydrogen catalytically. It also gives precipitate with ammonical cuprous chloride. Thus it should be a terminal alkyne.
Molecular formula of the chlorocompound (R – Cl) = 74.5
Molecular wt. is 74.5 – 35.5 = 39
R = C3H3
Thus A is ClH2C—CC—H
Question 9 : Identify (a) the chiral compound C, C10H14, that is oxidized with alk. KMnO4 to Ph COOH, and (b) the achiral compound D, C10H14, inert to oxidation under the same conditions.
Solution: Given

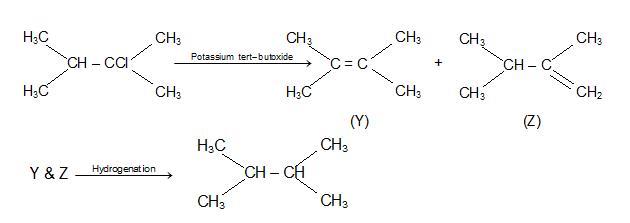
Question 10: An alkylhalide, X, of formula C6H13Cl on treatment with potassium tertiary butoxide gives two isomeric alkenes Y and Z (C6H12). Both alkenes on hydrogenation gives 2, 3-dimethylbutane predict the structures of X, Y and Z.
Solution: An alkyl halide X(C6H13Cl) gives two isomeric alkenes Y and Z. Since it is forming two isomeric alkane an dehydrohalogenation reaction, Cl should not be at the terminal alkyl group., The reaction are
?Click here to access the Organic Chemistry Revision Notes and IIT JEE Chemistry Syllabus
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More
