Colloidal State

While studying the diffusion of solutions through an animal membrane, Thomas Graham (1861) observed that certain substances such as sugar, urea, sodium chloride etc. in the dissolved state passed through the membrane, while the solutions of substances such as glue, gelatin, gum Arabic etc. did not. This observation led him to classify the soluble substances into two categories:
-
Crystalloids : Crystalloids were those substances which could be obtained in crystalline form and whose solutions were able to pass through an animal membrane.
-
Colloids : Colloids were those substances which were amorphous in nature and whose solutions were unable to pass through the membrane.
However, it was soon realized that the classification of dissolved substances made by Graham was not tenable because certain substances could act both as crystalloids and colloids.
Later on it was found that the diffusibility of crystalloids and non-diffusibility of colloids through an animal membrane was due to the difference in the size of their particles. Crystalloids formed smaller particles in solutions and therefore passed through the membrane. On the other hand, colloids formed larger particles (larger than the dimensions of the pores of the membrane) in solutions and were unable to pass through the membrane.
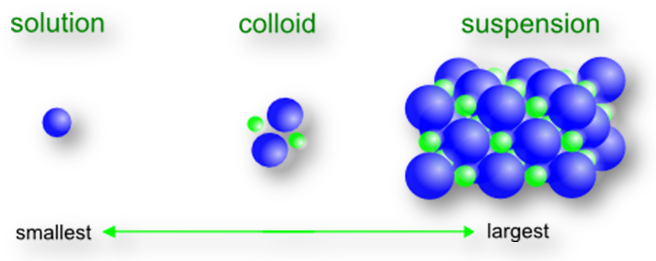
On the basis of the size of particles, the systems containing dispersed particles can be divided into following three categories.
-
True solutions: True solutions are homogeneous system and have the size of dispersed particles less than 1 nm, i.e. 10-9. The particles of solute present in a true solution are either single molecules or ions and are homogeneously distributed throughout the solutions.
These particles are invisible and cannot be seen even with a microscope. Due to very small size of dispersed particles, true solutions pass through ordinary filter paper as well as through animal membranes. Sodium chloride, sugar, urea etc. form true solutions in water.
-
Colloidal solutions: Colloidal solutions are heterogeneous systems and have the size of dispersed particles lying between 1 nm-1000 nm (i.e. 10-9-10-6 m). The particles in a colloidal solution are thus larger particles and are referred to as colloidal particles. Although colloidal particles are larger in size, yet they are not large enough to be seen with naked eye. However, they can be seen with the help of an ultra microscope. Colloidal solutions can pass through ordinary filter paper but not through an animal membrane, Gum Arabic, gelatin, glue etc. form colloidal solutions when dispersed in water.
-
Suspensions: Suspensions are also heterogeneous system and have still larger particles. The size of particles present in a suspension is more than 1000 nm (i.e., >10-6m). These particles are either visible to naked eye or can be seen under a microscope. The suspensions neither pass through an animal membrane nor through an ordinary filter paper. Stirred muddy water is an example of suspensions.
From the above discussion it is clear that colloidal solutions are intermediate of true solutions and suspensions. Colloidal solutions are not only formed by certain specific substances as mentioned above but they can be obtained from any substance by subdividing or aggregating its particles in the size range 1 nm-1000 nm. Practically, all substances can be made to exist in colloidal form. Therefore instead of talking of colloidal solution, it would be more appropriate to talk of the colloidal state of matter.
|
Definition of Colloidal State of Matter of Colloidal Solutions |
|
The colloidal state matter is the state in which the size of the particles lies in between 1nm (10-9m or 104) and 1000nm (10-6) and the systems consisting of dispersed particles in this range are called colloidal systems. |
|
The important characteristics of true solutions, colloidal solutions and suspensions |
||||
|
S.No. |
Property |
True solution |
Colloidal solution |
Suspension |
|
1. |
Particle size |
Less than 1 nm (i.e. 10-9m) |
Between 1 nm-1000nm (i.e. 10-9m-10-6m) |
Greater than 100nm (i.e. 10-6m) |
|
2. |
Nature |
Homogeneous |
Heterogeneous |
Heterogeneous |
|
3. |
Visibility of particles |
Invisible |
Visible under ultra-microscope |
Visible to naked eye or under microscope |
|
4. |
Appearance |
Transparent |
Generally transparent but may show translucence |
Opaque |
|
5. |
Filterability |
Passes easily through ordinary filter paper as well as animal membranes |
Passes easily through ordinary filter paper but not through animal membranes |
Does not pass either through ordinary filter paper or through animal membranes. |
|
6. |
Setting of particles under gravity |
Particles do not settle |
Colloidal particles do not settle under gravity. However, they can be made to settle under high speed centrifugation |
Particles settle on standing |
|
7. |
Diffusion of particles |
Diffuses rapidly |
Diffuses slowly |
Does not diffuse |
|
8. |
Scattering of light by particles (Tyndall effect) |
Does not scatter light |
Scatters light and exhibits Tyndall effect |
Tyndall effect may be exhibited |
 Dispersed Phase and Dispersion Medium:
Dispersed Phase and Dispersion Medium:
A colloidal system consists of two phase, viz.
-
Dispersed Phase: The phase which is dispersed or scattered through the dispersion medium is called Dispersed phase or discontinuous phase.
-
Dispersion Medium: The phase in which the scattering is done is called the dispersion medium or continuous medium.
Each of the two phases constituting a colloidal system may be a gas, a liquid or a solid. In milk, for example, the fat globules are dispersed in water. Hence fat globules form a dispersed phase and water is the dispersion medium. The term sol is applied to the dispersion of solid in liquid, solid or gaseous medium.
The dispersion of a solid (dispersed phase) in a liquid (dispersion medium) is called colloidal solution.
The dispersion of a solid (dispersed phase) in a gas (dispersion medium) is called solid aero sol. When a liquid is dispersed in another liquid, the resulting system is called an emulsion. When colloidal system becomes fairly rigid, it is refereed to a gel.
Examples |
|
Question 1: What are Colloids? Solution: A colloid can be dined as a heterogeneous system in which one substance is dispersed (dispersed phase) as very fine particles in another substance called dispersion medium. Colloids are the intermediate system between a solution and a suspension. The size of colloidal particles is between 1 nm and 1000 nm. The colloidal particles remain suspended in the medium and do not settle down under the influence of gravity. Milk and ink are the two examples of colloids _____________________________________________________ Question 2: What is the difference between colloids and suspension? Solution:
_____________________________________________________________ Question 3: Comment on the statement that “colloid is a state of matter and not substance” Solution A colloid is not a substance but a state of the substance which is dependent on the size of the particle. A colloid is formed when the size of the solute particle lies between 1 nm and 1000 nm. |
General Physical Properties of Colloidal Solutions
The important properties of colloidal solutions are described below. 
-
Heterogeneity: Colloidal solutions are heterogeneous in nature and consist of two phases-dispersed phase and dispersion medium.
-
Visibility of dispersed particles: Although colloidal solutions are heterogeneous in nature, yet the dispersed particles present in them are not visible to the naked eye and they appear homogenous. This is because colloidal particles are too small to be visible to the naked eye.
-
Filterability: Due to very small size, the colloidal particles pass through an ordinary filter paper. However, they can be retained by animal membranes, cellophane membrane and ultrafilters.
-
Stability: Lyophilic sols in general and lyophobic sols in the absence of substantial concentrations of electrolytes are quite stable and the dispersed particles present in them do not settle down even on keeping. However, on standing for a long time, a few colloidal particles of comparatively larger size may get sedimented slowly.
-
Colour: The colour of a colloidal solution depends upon the size of colloidal particles present in it. Larger particles absorb the light of longer wavelength and therefore transmit light of shorter wavelength. For example, a silver so having particles of size 150nm appears violet, whereas that having particles of size 60nm appears orange yellow.

Question 1: According to Thomas Graham those substances which were amorphous in nature and whose solutions were unable to pass through the membrane were defined as
a. crystals
b. crystalloids
c. colloids
d. emulsions
Question 2: Colloidal solutions are
a. homogeneous system
b. heterogeneous systems
c. true solutions
d. suspension
Question 3: Particles in the size of colloids range
a. 1 nm-1000 nm
b. 100 nm-100 nm
c. 1000 nm-10000 nm
d. 1 nm-100 nm
Question 4: Colloidal solution
a. does not diffuse
b. does not scatter light
c. is homogeneous
d. exhibits Tyndall effect

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
c |
b |
a |
d |
Related Resources
-
You can also refer to syllabus of chemistry for IIT JEE
-
Click here to have a look at past year papers of IIT JEE
-
You can look here for enzyme catalysis
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free