Mole Concept
Table of Content |
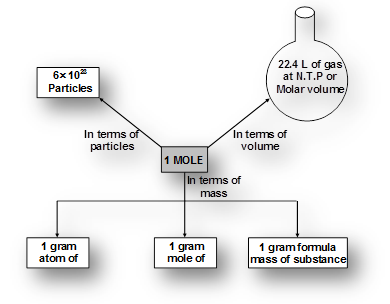
What is Mole?
Atoms and molecules are too small to count. To solve this problem their numbers are expressed in terms of Avogadro’s number (NA = 6.023 1023). Mole is the number equal to Avogadro’s number just like a dozen is equal to 12, a century means 100, a score means = 20.
Mole can be defined as a unit which represents 6.023 x1023 particles of same matter.
A mole (symbol mol) is defined as the amount of substance that contains as many atoms, molecules, ions, electrons or any other elementary entities as there are carbon atoms in exactly 12 gm of . The number of atoms in 12 gm of
. The number of atoms in 12 gm of  is called Avogadro’s number
is called Avogadro’s number .
.

One atomic mass unit (amu)
The number of moles of a substance can be calculated by various means depending on data available, as follows.
-
Number of moles of molecules

-
Number of moles of atoms

-
Number of moles of gases
 (Standard molar volume at STP = 22.4 lit)
(Standard molar volume at STP = 22.4 lit) -
Number of moles of particles e.g. atoms, molecules ions etc

-
Mole fraction = fraction of the substance in the mixture expressed in terms of mol is called its mol fraction (X)
E.g. for a mixture of substance A & B
![]() (n terms of denote number of moles)
(n terms of denote number of moles)
![]()
Refer to the following video for mole concept
|
Solved Examples |
|
Question 1: Calculate the mass of (i) an atom of silver (ii) a molecule of carbon dioxide. Solution:
= 6.022 x 1023 atoms 6.022 x 1023 atoms of silver have mass = 108g Mass of one atom of silver
= 6.022 x 1023 molecules Thus, 6.022 x 1023 molecules of CO2 has mass = 44 g 1 molecule of CO2 has mass = 7.307 ´ 10-23 g ______________________________________________________________ Question 2: Calculate the number of molecules present
Solution:
= 12 x 12 + 22 x 1 + 11 x 16 = 342 amu = 6.022 x 1023 molecules Now 342 g of cane sugar contain 6.022 x 1023 molecules. 34.2 g of cane sugar will contain = 6.022 x 1022 molecules
Mass of 1 litre of water = Volume x density = 1000 x 1 = 1000 g Now 18 g of water contains = 6.022 x 1023 molecules. 1000 g of water will contain = = 3.346 x 1025 molecules
Mass of 1 drop of water = 0.05 g Now 18 g of H2O contain = 6.022 x 1023 molecules. 0.05 g of H2O will contain = ________________________________________________ Question 3: Calculate the number of moles in each of the following:
Solution:
Thus 98 g of H2SO4 = 1 mole of H2SO4 392 g of H2SO4 =
i.e. 22.4 litres of CO2 at STP = 1 mole 44.8 litres of CO2 at STP = = 2 moles CO2
6.022 x 1023 molecules = 1 mole of oxygen molecules.
i.e. 27 g of aluminium = 1 mole of Al 9 g of aluminium =
1 mole of Fe = 56 g of Fe 106 g of Fe = = 1.786 |
Equivalent Weight
Equivalent weight of a substance (element or compound) is defined as “The number of parts by weight of it, that will combine with or displace directly or indirectly 1.008 parts by weight of hydrogen, 8 parts by weight of oxygen, 35.5 parts by weight chlorine or the equivalent parts by weight of another element”.
Equivalent weight of any substance depends on the reaction in which it takes part.
Equivalent weight is a relative quantity so it is unit less. When equivalent weight of a substance is expressed in grams, it is called Gram Equivalent Weight (GWE).
Calculation of Equivalent Weight
Equivalent weight = Molar mass / Valence factor
-
Valence factor for base = acidity of base
-
Valence factor for acid = basicity of acid
-
Valence factor for element = valency
 Titration
Titration
Titration is a procedure of determining the concentration of unknown solution with the help of solution of known concentration.
In this procedure of determining the concentration of solution A by adding carefully measured volumes of a solution of known concentration B until the reaction of A with B is just complete.
Law of equivalence:
The fundamental basis of titration is the Law of Equivalence which states that
“at end point of a titration, volumes of the two titrants reacted have the same number of equivalents or mili equivalents”
Acid Base Titration:
One gram equivalent of acid neutralized by one gm equivalent of base. It means
One equivalent of Acid = One Equivalent of Base
Acid [N1V1] = Base [N2V2]
[Gram equivalent = Normality x Volume]
|
Quesion Find the number of milli equivalents of H2SO4 present in 10 mL of N/2 H2SO4 solution. Solution Milli equivalents = Normality x Volume (mL) = ½ X 10 = 5 milli equivalent of H2SO4 |
Limiting Reactant
The reactant which is totally consumed during the course of reaction and when it is consumed reaction stops.
The concept of limiting reactant is applicable to reaction other than monomolecular i.e., when more than one type reactant involved. For example![]() . These is no limiting reactant.
. These is no limiting reactant.
To determine the limiting reagent amount of all reactants and mole ratio of reactants must be known. If the ratio of moles of reactant A with respect to reactant B is greater than the ratio of the moles of A to moles of B for a balanced chemical equation then B is the limiting reactant.
All other terms like left (unused) mass of other reactant, amount of formed product can be known stoichiometrically by knowing the amount of limiting reactant.


Question 1: The largest number of molecules is in:
a. 28 g of CO
b. 46 g of C2H5OH
c. 36 g of H2O
d. 54 g of N2O5
Question 2: The number of molecules in 89.6 litre of a gas at NTP are:
a. 6.02 × 1023
b. 2 × 6.02 × 1023
c. 3 × 6.02 × 1023
d. 4 × 6.02 × 1023
Question 3: The total number of protons in 10 g of calcium carbonate is:
a. 3.0115 × 1024
b. 1.5057 × 1024
c. 2.0478 × 1024
d. 4.0956 × 1024
Question 4: What is equivalent weight of NaCl?
a. 58.5
b. 12.12
c. 13.45

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
c |
d |
a |
a |
Related Resources
-
You can also refer to past year papers of IIT JEE
-
Look here for the syllabus of chemistry for IIT JEE
-
Click here to refer laws of chemical combination
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free

 for a compound
for a compound , y moles of A = x moles of B
, y moles of A = x moles of B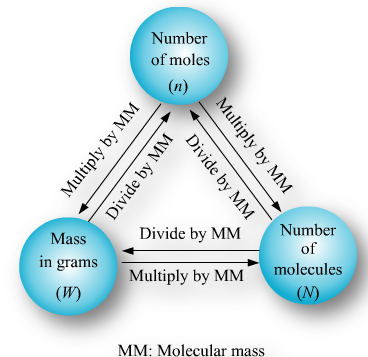







 = 4 moles of H2SO4
= 4 moles of H2SO4



 104 moles
104 moles

