General Characteristics of Transition Elements
Table of Content |
Metallic Character
-
All the transition elements are metals; this is because the number of electrons in outermost shell is only 2.
-
Transition metals are hard, malleable and ductile due to presence of strong metallic bonds.
-
Transition metals crystallize in all the three face centred cubic (fcc), hexagonal close packed (hcp) and body centred cubic (bcc) crystals.
-
Transition metals of VIII and IB Groups are softer and more ductile as compare to the other transition metals.
-
Along with metallic bonding, transition metals also show covalent bonding due to presence of unfilled d-orbitals.
-
As transition elements are metals so they good conductors of heat and electricity.
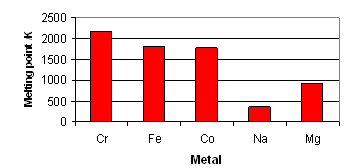
Melting and Boiling Point
-
Transition metals usually have very high value of melting and boiling points due to presence of strong metallic bonds.
-
Zn, Cd and Hg metals have lower metaling and boiling points as they have completely filled d orbitals because of which no unpaired electron is available. Because of unavailability of unpaired electrons, these metals do not undergo covalent bonding. Rest of the transition metals does have metallic as well as covalent bonding.
-
Metals towards the middle of each transition series have highest melting point.?
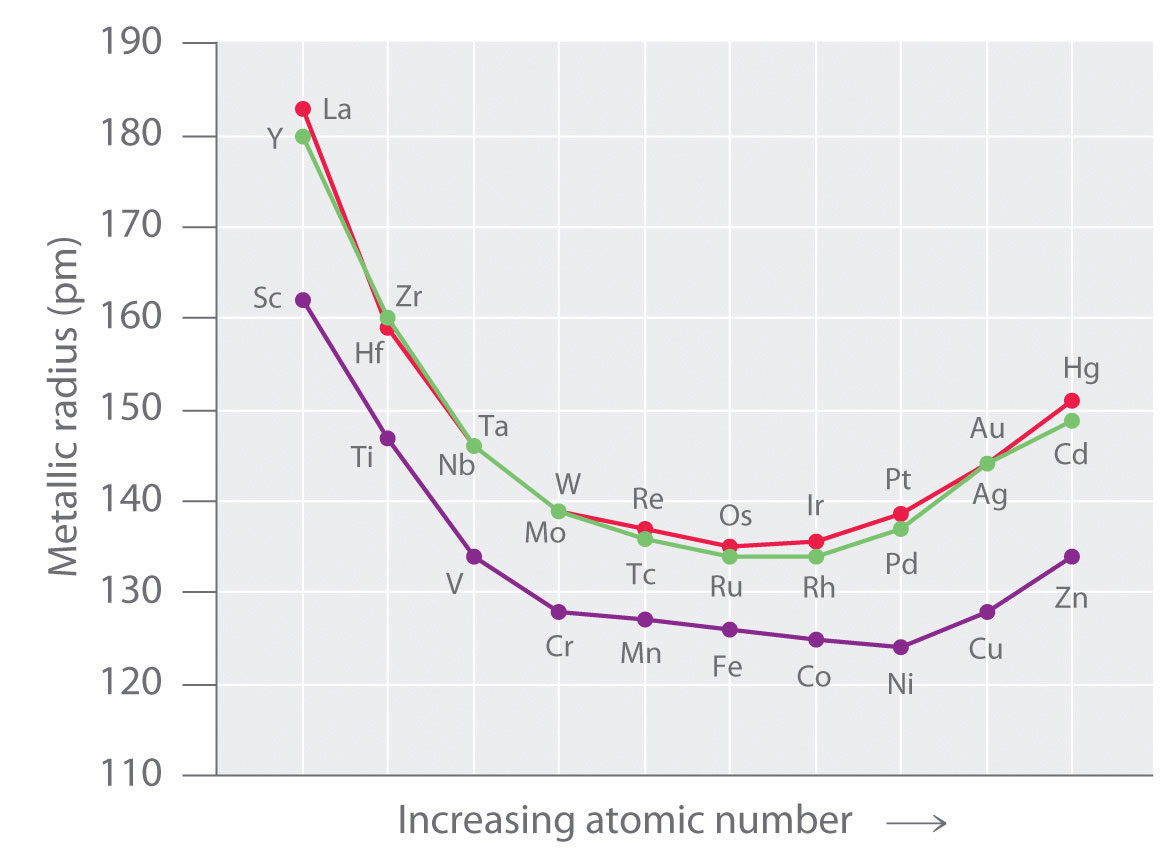
Atomic (Covalent) and Ionic Radii
-
The covalent radii of the elements decrease from left to right across a row in the transition series. This is because of the poor screening by the d electrons due to which, the nuclear charge attracts all of the electrons more strongly, hence a contraction in size occurs.
-
The atomic radii for the elements from Cr to Cu are very close to one another. This closeness in atomic radii is due to the shielding of outer 4s electrons by 3d electrons from the inward pulls of nucleus. As a result of these two opposing effects, the atomic radii do not alter much on moving from Cr to Cu.
-
The elements in the first group in the d-block show the excepted increase (due to the addition of extra shell) in size Sc → Y → La. However in the subsequent groups there is an increase between first and second members, but hardly any increase between second and third elements. This is due to lanthanide contraction (discussed in f-block elements).
The ionization energy (IE)
-
The first ionization energy of transition elements are higher than those of s-block elements but lower than p-block elements.
-
In a particular transition series, ionization energy although increases gradually as we move from left to right but this increase is not appreciable.
-
The increase in ionization energy is due to increase in nuclear charge, the effect of increase in nuclear charge is partly balanced by the increase in screening effect. Consequently, the increase in ionization energy along the period of d-block elements is very small.
-
In transition elements, on moving along the period, the addition of the extra electron in the (n-1) d level takes place. This electron provides a screening effect and shields the outer ns electrons from the nucleus pull.
-
Because of this shielding effect of d electrons, the effect of nuclear charge (effective nuclear charge) on outer ns electrons is somewhat less than the actual nuclear charge.
-
Thus the effects of the increasing nuclear charge and the shielding effect created due to the expansion of (n-1)d orbital oppose each other.
-
On account of these counter effects, the ionization potentials increase rather slowly on moving in a period of the first transition series
First Ionization Energy
-
The first ionization energy for the first four 3d series elements (Sc, Ti, V and Cr) arte almost same i.e. differ only slightly from one another.
-
The values of first ionization energy for Fe, Co, Ni and Cu are also very close to one another.
-
The value of first ionization energy for Zn is considerably high due to extra-stability of completely filled 3d10 level.
-
The second ionization emerges increases more or less regularly with the increase of atomic number.
-
The value of second ionization energy for Cr and Cu are higher than those of their neighbors. This is due to the fact that the electronic configurations of Cr+ and Cu+ ions have extra stable 3d5 and 3d10 levels.
-
There is a sudden fall in the values of ionization potentials in going from II B (Zn-group elements) to IIIA sub-group. This is because in case of IIIA group elements the electron to be removed is from a 4p-orbital which is incompletely filled, while in case of the II B group elements, the electron to be removed is from completely filled 4s-orbital which required extra energy
Colour
Many compounds of transition elements are coloured in contrasts to those of s and p block elements.In compound state due to the surrounding groups (ligands), the d-orbitals of transition elements are not degenerate but split into two groups of different energy.
Thus it is possible to promote electrons from one group to another group. This corresponds to fairly small amount of energy difference and so light is absorbed in visible region. Some compounds of transition metals are white, for example ZnSO4 and TiO2. In these compounds it is not possible to promote the electrons within the d-level.
Formation of Alloys
Alloys are homogenous solid solutions of two or more metals obtained by melting the components and then cooling the melt. These are formed by metals whose atomic radii differ by not more than 15% so that the atoms of one metal can easily take up the positions in the crystal lattice of the other. Since transition metals have similar atomic radii, they form alloys very readily.
Interstitial Compounds
-
Sometimes transition metals form non stoichiometry compounds. These are compounds of indefinite structure and proportions. For example Fe0.94O. It is mostly due to the variable valency of transition elements. Sometimes, non stoichiometry is caused by defects in the solid structures.
-
Transition metals form number of interstitial compounds, in which they take up atoms of small size e.g. H, C and N in the vacant spaces in their lattices. The presence of these atoms result in decrease in malleability and ductility of the metals but increases their tensile strength.
Variable Oxidation State
They show variable oxidation states unlike s and p block elements.The oxidation states changes in units of one, e.g. Fe2+ and Fe+3, Cu+1 and Cu+2.
Scandium can have an oxidation number of (+II) if both s electrons are used for bonding and (+III) when two s and one d electrons are involved. Similarly all the elements show variable oxidation states depending upon the number of electrons available for bonding in their s and d sub-shells.
Refer to the following video for characteristics of transition elements
Catalytic Property
Many transition metals and their compounds have catalytic properties. For e.g. V2O5, Fe, FeCl3, Ni, Pd etc. This is due to following reasons
-
Variable oxidation state: Due to variable oxidation state they form unstable intermediate compounds and provide a new path with lower activation energy for the reaction (Intermediate compound formation theory)
-
Large Surface area: Finely divided transition metals or their compounds provide a large surface area for adsorption and the adsorbed reactants react faster due to the closer contact.
Complex Formation
The transition elements have an unparalleled tendency to form coordination compounds with the Lewis bases, which are called as ligands.
CO3+ + 6NH3 → [CO(NH3)6]3+
Fe2+ + 6CN– → [Fe(CN)6]4–
s and p block elements form very few complexes. The reason transition elements are so good at forming complex is that they have small, highly charged ions and have vacant low energy orbitals to accept lone pairs of electrons donated by ligands.
Transition element/compound |
Catalystic Action |
|
TiCl3 |
Used as Ziegler – Natta catalyst |
|
V2O5 |
Converts SO2 to SO3 in the contact process for making H2SO4 |
|
MnO2 |
Used as a catalyst to decompose KClO3 to give O2 |
|
Fe |
Used in Haber – Bosch process for making NH3 |
|
FeCl3 |
Production of CCl4 from CS2 and Cl2 |
|
FeSO4 & H2O2 |
Fenton’s reagent |
|
PdCl2 |
Wacker process for the following conversion :C2H2 + H2O + PdCl2 → CH3CHO + 2HCl + Pd |
|
Pd |
For hydrogenation (Phenol → Cyclohexanone) |
|
Pt/PtO |
Adams catalyst used for reduction |
|
Pt |
SO2 → SO3 contact process |
|
Cu |
In manufacture of (CH3)2SiCl2 |
|
Oxidation of cyclohexanol |
|
|
CuCl2 |
Deacon process for making Cl2 from HCl |
|
Ni |
Raney nickel |
Variable Oxidation State
Transition elements show variable oxidation states unlike s and p block elements.The oxidation states changes in units of one, e.g. Fe2+ and Fe+3, Cu+1 and Cu+2.
Scandium can have an oxidation number of (+II) if both s electrons are used for bonding and (+III) when two s and one d electrons are involved. Similarly all the elements show variable oxidation states depending upon the number of electrons available for bonding in their s and d sub-shells.
Transition metals have unique property to show variable oxidation state. This property arises from the fact that the energy levels of 3d, 4d and 5d orbitals are very close to those of 4s, 5s and 6s orbitals respectively and, therefore, electrons from both ns and (n-1)d orbitals can be used, infect they are used, for the formation and bonds by transition metals.
Minimum oxidation state: All the transition elements except Cr, Cu, Ag, Au and Hg which have a minimum oxidation state of +1 exhibit a minimum oxidation state of +2.
Maximum oxidation state: Each of the elements in groups III B to VII B can show the maximum oxidation state equal to its group number. For example, Cr in group VIB shows a maximum oxidation state of +6 in Cr2O72– ion.
Most of the elements in VIII group show a maximum oxidation state equal to + 6. However, Ru and Os have a maximum oxidation state equal to +8 which is the highest oxidation state shown by any element.
Relative stability of various oxidation states:
-
The relative stabilities of various oxidation states of 3d-series elements are due to the extra stability of 3d0, 3d5 and 3d10 configurations.
-
Ti4+ (3d0) is more stable than Ti3+ (3d1)
-
The higher oxidation state of 4d and 5d series elements are generally more stable than those of the elements of 3d series, e.g., Mo, Tc (4d series elements) and W, Re (5d-series elements) s.
-
The highest oxidation states of second and third row elements are encountered in compounds containing the more electronegative elements viz. F, O and Cl
Magnetic Property
On the basis of behaviour in a magnetic field, substance are classified as paramagnetic, diamagnetic and ferromagnetic. Those substance which are attracted by the applied magnetic field are called paramagnetic where as those which are repelled by the magnetic field are called diamagnetic. Substances which are very strongly attracted by the applied field are called ferromagnetic.
Paramagnetism is a property due to the presence of unpaired electrons. Thus most of the transition metals are paramagnetic. As the number of unpaired electrons increases, the paramagnetic character also increases.
The magnetic moment is calculated from the following formula μ = √n(n+2) BM where n is the number of unpaired electrons and B. M stands for Bohr magneton.
Density
Because of small size of their atoms and strong metallic bonding the density and hardness of transition elements are high.? Except for mercury, which is a liquid at room temperature all other elements are solid metals exhibiting all the characteristics of a metal.
Illustrations |
|
Question 1: K2PtCl6 is a well known compound whereas corresponding Ni compound is not known. State a reason for it. Solution: This is because Pt4+ is more stable than Ni4+ has the sum of four ionization energies of Pt is less than that of Ni. ________________________________________________________________________ Question 2: Why do transition elements show variable oxidation states? ________________________________________________________________________ Question 3: Explain briefly how +2 state becomes more and more stable in the first half of the first row transition elements with increasing atomic number. or Compare the stability of +2 oxidation state of the elements of the first transition series. Solution: The sum IE1 + IE2 increases. As a result the standard reduction potentials (E0) becomes less and less negative. Hence the tendency to form M2+ ion decreases. The greater stability of +2 state for Mn is due to half-filled d-subshell (d5), that for zinc is due to completely filled d-subshell (d10) and half that for nickel is due to highest negative enthalpy of hydration. ________________________________________________________________________ Question 4: Why Zn2+ salts are white while Ni2+ salts are blue? ________________________________________________________________________ Question 5: Why Zn2+ salts are white while Cu2+ salts are blue? Solution: Reason same as above. ________________________________________________________________________ Question 6: Giving reasons indicate which one of the following would be coloured? Cu+, VO2+, Sc3+, Ni2+ (At. Nos Cu = 29, V = 23, Sc = 21, Ni = 28) Solution: Ni2+ due to incompletely filled d-orbitals. |

Question 1: Transition elements..
a. form colourless compounds
b. do not form complexes
c. are metals and non metals both.
d. show variable oxidation states.
Question 2: Which of the following statements is incorrect?
a. Ti4+ is more stable than Ti3+
b. Mn2+ is more stable than Mn4+
c. The higher oxidation state of 3d series elements are generally more stable than those of the elements of 5d series.
d. The highest oxidation states of second and third row elements are encountered in compounds containing the more electronegative elements
Question 3: CO3+ + 6NH3 →
a. [CO(NH3)3]3+
b. [CO(NH3)5]2+
c. [CO(NH3)6]3+
d. [CO(NH3)2]2+
Question 4: Which of the following statements is incorrect?
a. All the transition elements are metals
b. Transition metals are hard, malleable and ductile due to presence of strong metallic bonds.
c. Transition metals crystallize in all the three face centred cubic (fcc), hexagonal close packed (hcp) and body centred cubic (bcc) crystals.
d. Transition metals of VIII and IB Groups are harder and less ductile as compare to the other transition metals.
|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
d |
c |
c |
d |
Related Resources
-
Click here to refer past year papers of IIT JEE
-
Click here for the syllabus of chemistry for IIT JEE
-
You can also refer to d and f block elements.