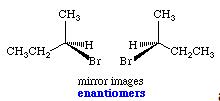
Enantiomers, Diastereomers & Meso Compounds
Table of Content |
If an organic molecule contains more than one chiral carbons then the molecule may be chiral or achiral depending whether it has element of symmetry or not.
Elements of Symmetry
If a molecule have either
a) a plane of symmetry, and / or
b) centre of symmetry, and / or
c) n-fold alternating axis of symmetry
If an object is superimposable on its mirror image; it cannot rotate PPL and hence optically inactive. If an object can be cut exactly into two equal halves so that half of its become mirror image of other half., it has plane of symmetry.
Centre of symmetry: It is a point inside a molecule from which on travelling equal distance in opposite directions one takes equal time
Thus, if an organic molecule contains more than one chiral carbons but also have any elements of symmetry, it is superimposable on its mirror - image, cannot rotate PPL and optically inactive. If the molecule have more than one chiral centres but not have any element of symmetry, it must be chiral.
What are Enantiomers?
Stereoisomers which are related to each other as mirror images are called enantiomers. Enantiomers can contain any number of stereogenic centers, as long as each center is the exact mirror image of the corresponding center in the other molecule.
If one or more of these centers differs in configuration, the two molecules are no longer mirror images, but are totally different chemical compounds with differing physical and biological properties. Stereoisomers which are not enantiomers are called diastereomers.
For a molecule with multiple chiral centers, the number of possible diastereomers is given by Van't Hoff rule : x = 2n
Where x is the number of possible isomers and n is the number of stereogenic centers. Thus, for molecules with two stereogenic centers there are four possible stereoisomers. For cholesterol, with eight stereogenic centers, there are 256 possible stereoisomers, etc.
A third type of stereoisomer which must be considered is a meso compound. A meso compound contains at least two stereogenic centers, yet the molecule itself is not chiral. This is because meso compounds contain an internal plane of symmetry; the molecule can be split by an imaginary mirror so that all atoms on one side of the mirror are the exact reflection of the atoms on the other side. This can be seen below for cis-1,2-dimethylcyclopentane; there are two chiral centers in the molecule since the two carbons labeled with the red asterisk are each bonded to four different groups. A mirror placed through the molecule, along the plane indicated by the dashed line, will exactly bisect the molecule with all groups exactly reflected by their counterparts on the other side of the "mirror".
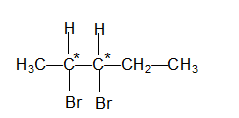
Stereoisomerism in 2,3-dibromopentane?
The structural formula of 2,3-dibromopentane is
The molecule contains two chiral carbons and hence according to Van't Hoff rule the total number of optical isomers should be 2n = 22 = 4 and it is. The four optical isomers are.
I,II,III and IV are four stereoisomers of 2,3-dibromopentane.
I and III are enantiomers.
III and IV are also enantiomers
What is relation between I and III; or I and IV; or II and III; or II and IV?
All these pairs are diastereomers, stereoisomers which are not mirror - image of each other are called diastereomers.
Therefore,
I and IV are diastereomers
II and III are diastereomers
II and IV are diastereomers
Stereoisomerism in Tartaric Acid
The IUPAC name of tartaric acid is 2,3 - dihydroxy butandioic acid. The structural formula is
HOOC-*CHOH-*CHOH-COOH
The molecule contains two chiral carbon and the number of optical isomers should be 2n=22=4; but number of optical isomers reduces to 3 because one molecule has plane of symmetry.
III and IV are same
Rotation of IV by 180° yield III.
I and II are enantiomers
III is meso-form of tartaric acid.
A meso compound is one which is optically inactive although have more than one chiral carbons.
Number of Optical Isomers
Number of possible optical isomers in compounds containing different no. of asymmetric atoms.
- The molecule has no symmetry
The no. of d and l – forms a = 2n
n = no. of asymetric atoms
The no. of meso l- forms m = 0
Total no. of optical isomers = a + m = 2n
- The molecule has symmetry
The no. of d and l forms a = 2n -1
Meso forms m = 2n–1
Total = a + m
It is add a = 2n–1 , m = 2(n-1)/2
Difference Between Racemic Mixture and Meso Compound
A racemic mixture contains equimolar amounts of enantiomers. It is optically inactive due to external compensation. It can be resolved into optically active forms. A meso compound is optically inactive due to internal compensation.
Optically active compounds having no chiral carbon
The presence of chiral carbon is neither a necessary nor a sufficient condition for optical activity, since optical activity may be present in molecules with no chiral atom and since molecules with two or more chiral carbon atoms are superimposable on their mirror images and hence inactive.
(i) Any molecule containing an atom that has four bonds pointing to the corners of a tetrahedron will be optically active if the four groups are different.

(ii) Atoms with pyramidal bonding might be expected to give rise to optical activity if the atom is connected to three different groups, since the unshared pair of electron is analogous to a fourth group.
Many attempts have been made to resolve such compounds, but until recently all failed because of umbrella effect, also called pyramidal inversion. The umbrella effect is rapid oscillation of the unshared pair form one side of XYZ plane to the other.
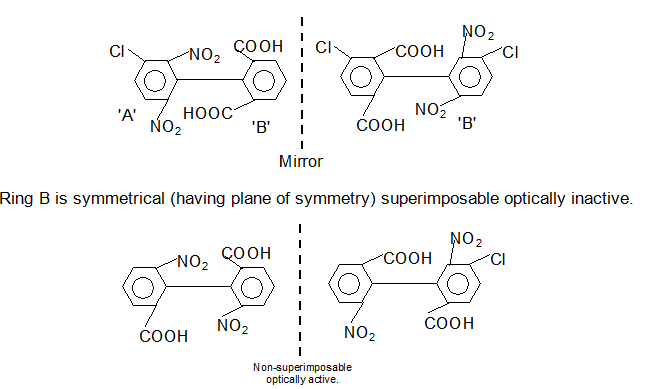
(iii) Biphenyls, containing four large groups in the ortho position so that there is restricted rotation, are optically active if the rings are asymmetrical.
If either or both rings are symmetrical, the molecule has plane of symmetry and optically inactive.
Allenes, with even number of cumulative double bonds are optically active if both sides are dissymmetric.

Specific Rotation
The specific rotation [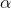 ]
] is an inherent physical property of an enantiomer, which varies with the solvent used, temp (in °C) and wavelength of the light used. It is calculated from the observed rotation a as follows.
is an inherent physical property of an enantiomer, which varies with the solvent used, temp (in °C) and wavelength of the light used. It is calculated from the observed rotation a as follows.
[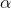 ]
] =
= 
Where l = length of tube in decimeters (dm)
C = Concentration in gram cm–3, for a solution density in gcm–3, for a pure luqid
Click here to refer the Revision notes on Organic Chemistry, IIT JEE Organic Chemistry Syllabus and Chemistry books
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More