Phosphorus Halides
Table of Content |
Phosphorous structures two sorts of halides, to be specific, PX3 and PX5. Here, X speaks to a halogen, which might be fluorine, chlorine, bromine or iodine. Of these, chlorides are more normal. These chlorides are covalent in nature.
Phosphorus Trichloride
It is a dry, oily, sleek fluid and exceedingly lethal compound. The compound has triangular pyramidal shape where phosphorus exhibits SP3 hybridization.
Figure A
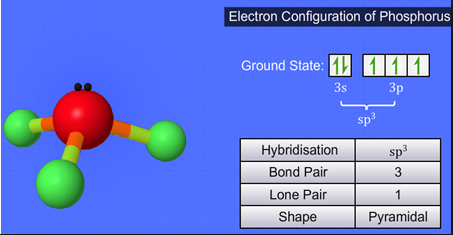
As described in Fig. A, Phosphorus with its SP3 orbitals, which had only one electron, offering that electron to a p orbital electron from 3 chlorine atoms. The fourth sp3 orbital is full (a solitary lone pair) so it can't bond, however it repels alternate bonds creating the state of the particle to be a trigonal pyramidal.
 |
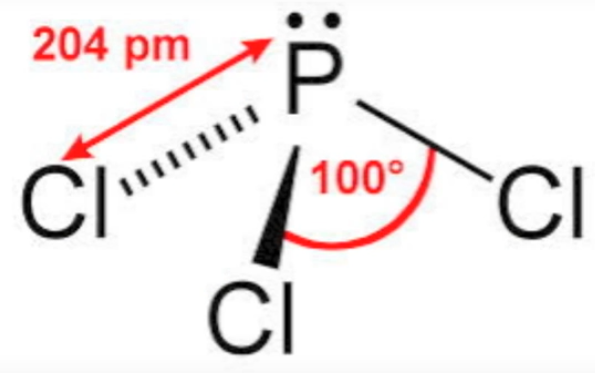 |
Fig. B: Electronic configuration of Phosphorus with structure details |
Fig. C: Bond angle and bond length of phosphorus trichloride |
Preparation:
-
At the point when dry chlorine proceeds over heated white phosphorus, phosphorus trichloride is obtained.
P4 + 6Cl2→4PCl3
-
At the point when thionyl chloride reacts with white phosphorus, phosphorus trichloride is obtained.
P + 8SOCl2 → 4PCl3 + 4SO2 + 2S2Cl2
Chemical Properties:
-
Phosphorus trichloride hydrolyses in nearness of dampness.
PCl3 + 3H2O → H3PO3 + 3HCl
3C2H5OH + PCl3 → 3C2H5Cl + H3PO3
Structure of PCl3:
The central phosphorus particle in PCl3 experiences sp3 hybridization with 3 bond sets and 1 lone pair of electrons. Because of this, it is pyramidal in shape. It goes about as a Lewis base because of its capacity to give its lone pair of electrons to other electron-lacking particles or atoms.
Phosphorus Pentachloride
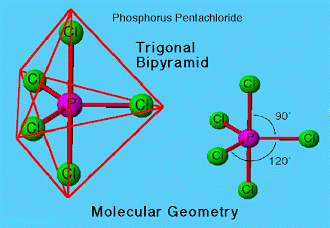
It is a yellowish white, water delicate solid. It is dissolvable in carbon tetrachloride, benzene, carbon disulfide, benzene, diethyl ether. It has a trigonal bipyramidal structure in vaporous and fluid stages. In solid state, it exists as an ionic solid,[PCl4]+[PCl6]– in which the cation, [PCl4]+is tetrahedral and the anion, [PCl6]– is octahedral.
From the figure, we recognize the arrangement of three tropical P-Cl bonds and two pivotal P-Cl bonds. Because of more prominent repulsion at hub positions in contrast with central positions the two axial bonds are longer than tropical bonds.
Preparation:
- When white phosphorus reacts with excess of dry chlorine, phosphorus pentachloride is produced.
P4 + 10Cl2→4PCl5
- It can also be prepared by the reaction of SO2Cl2 and phosphorus.
P4 + 10SO2Cl2→4PCl5+10SO2
Chemical Properties:
- In presence of damp air, phosphorus pentachloride hydrolyses to POCl3 which at long last changes over to phosphoric acid.
PCl5 + H2O → POCl3 + 2HCl POCl3 + 3H2O → H3PO4 + 3HCl
-
Upon heating, it sublimes and further breaks down to phosphorus trichloride if there should be an occurrence of more grounded heating.
PCl 5 → PCl3 + Cl2
- It reacts with finely partitioned metals affected by heat to create metal chlorides.
2Ag + PCl5 → 2AgCl + PCl3
- It reacts with natural compounds containing –OH group to create their chloro subordinates.
C2H5OH + PCl5 → C2H5Cl + POCl3 + HCl
Structure of PCl5:
The central phosphorus particle in phosphorus pentachloride experiences SP3d hybridisation. All the five electron combines in these hybrid orbitals are bond sets. The molecular geometry of the particle is trigonal bipyramidal.
Figure D
As described in Fig. D, After the five electrons are hybridized, they are five electrons of equivalent size and shape. They are five sp3d orbital electrons. Three frame a triangle (120° partition) in the center. One bond is above and one is underneath those 3. Here is the geometry of how it will bond with the 5 chlorine particles.
Trigonal bipyramidal geometry is watched for phosphorus pentachloride just in its fluid and vaporous state. It exists as a salt in the solid state.
Fig. E: Molecular geometry of phosphorus pentachloride
Uses of Phosphorus Halides
-
It is utilized as a chlorinating agent.
-
It is utilized for making water treatment agents, making organophosphorus pesticides, lube oil and paint added substances and so forth as an intermediate.
It is utilized in the preparation of phosphorus acid, chloroanhydrides and phosphoric acid subsidiaries as an intermediate.
Watch this Video for more reference
More Readings