Uses of Boron and Aluminium and their Compounds
Table of Content |
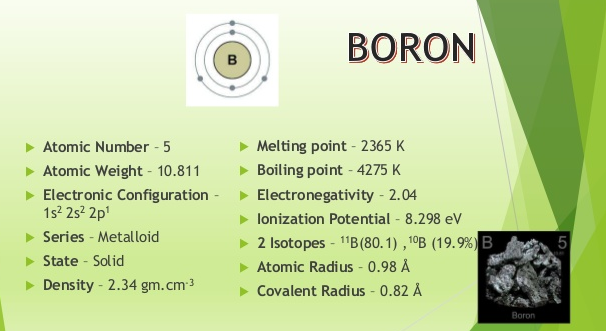
Fig. 1: Physical Properties of Boron
Discovery and Naming
The principal mention of boron compounds is found in a book by Persian chemist Rhazes (c. 865-c. 925). Alchemists concentrated the way of matter before present day chemistry was conceived. Rhazes characterized minerals into six classes, one out of which was the boraces, which included borax.
Borax was generally utilized by crafts individuals. It lessens the melting point of materials used to make glass. It was additionally used to melt the minerals of metals and for the isolation of the metals from those minerals.
In 1808, English scientist Humphry Davy (1778-1829) had recently figured out how to extract the most dynamic metals, for example, sodium and potassium. He was additionally taking a shot at a technique to expel boron from its compounds.
News of Davy's prosperity had headed out to France, where ruler Napoleon Bonaparte (1769-1821) became worried about the scientific notoriety of his nation. He requested bigger and better equipments for his researchers. He needed them to outperform Davy in his work on metals. This hardware was outlined particularly for two French physicists, Louis Jacques Thênard (1777-1857) and Joseph Louis Gay-Lussac (1778-1850).
Fig. 2: Above are the scientists credited with the discovery of Boron
Thenard and Gay-Lussac found another approach to separate boron from its compounds. They warmed boracic acid (otherwise called boric acid, H3BO3 ) with potassium metal to deliver boron in impure form. Thênard and Gay-Lussac were given acknowledgment for finding the new element. In 1892, French physicist Henri Moissan (1852-1907) discovered boron that was 98 percent immaculate.
The names borax and boracic acid most likely began as far back as the time period of Rhazes as buraq (in Arabic) or burah (in Persian).
Physical Properties
One of the strange properties of boron is the numerous physical structures, called allotropes, in which it happens. Allotropes are types of an element with various physical but similar chemical properties. One type of boron comprises of clear red gems with a thickness of 2.46 grams for every cubic centimeter. Another shape comprises of dark precious stones with a metallic appearance and a thickness of 2.31 grams for every cubic centimeter. Boron can likewise happen as a chestnut powder with no crystalline structure. The thickness of this powder is 2.350 grams for every cubic centimeter.
All types of boron have high melting points, from 2,200 to 2,300°C (4,000 to 4,200°F).
One property of unique significance is boron's capacity to retain neutrons. Neutrons are subatomic particles with no charge that happen in the core of almost all atoms. Boron molecules can assimilate countless neutrons. This makes boron valuable in the control bars of atomic reactors.
An atomic reactor is a gadget for creating energy from atomic splitting reactions. Atomic splitting is the procedure in which vast molecules are split, discharging a lot of energy and littler atoms. In an atomic reactor, it is basic that quite recently the correct number of neutrons is available. An excessive number of neutrons can make a splitting reaction gain out of power and excessive fewer neutrons lead the fission reaction to an end.
Control rods are long tubes pressed with boron (or some other element). The bars can be raised up and brought down up in the reactor. As the rods are brought down into the core, the boron assimilates neutrons, moderating the reaction.
Chemical Properties
Boron consolidates with oxygen in the air to shape boron trioxide (B2O3). Boron trioxide frames a thin film at first glance that averts any further reaction with oxygen.
Boron is not dissolvable in water. It ordinarily does not react with acids. In powder frame, it reacts with hot nitric acid (HNO3) and hot sulfuric acid (H2SO4). It likewise breaks up in liquid (liquefied) metals.
Boron reacts with concentrated H2SO4 and evolves Sulphur dioxide, SO2:
Occurrence in Nature
The plenitude of boron in the Earth's outside layer is assessed to be around 10 parts for each million. That spots it in about the center of the elements as far as their plenitude in the earth.
Boron never happens as a free element yet dependably as a compound. The most widely recognized minerals of boron are borax, or sodium borate (Na2B4O7); kernite (another type of sodium borate); colemanite, or calcium borate (Ca2BO11); and ulexite, or sodium calcium borate (NaCaB5O9). These minerals typically happen as white crystalline stores in regions of deserts. The two biggest world makers of boron compounds are Turkey and the United States. Moderate amounts originate from Argentina and China. About the greater part of the boron in the United States originates from three California areas: Kern, Inyo, and San Bernadino.
Uses of Boron
Fig. 3: Appearance of Boron
-
Metal borides are utilized as a part of nuclear reactors as defensive shields and control rods as a result of the high capacity of 10B isotope to ingest neutrons.
-
It is utilized as a part of steel industry for expanding the hardness of steel.
-
It is utilized as a semiconductor for making electronic gadgets.
-
Boron compounds are turning out to be progressively vital as rocket fills on account of their high energy/weight proportion.
-
Boron fibers are utilized as a part of making light composite materials for airships.
-
Boron is a basic element in plant digestion system.
-
Boron carbide filaments are hard however light and subsequently, are utilized for making bullet- proof vests.
Uses of Aluminum
Fig. 4: Appearance of Aluminium
-
Aluminum is utilized widely in industry and regular day to day existence.
-
It shapes compounds with Cu, Mn, Mg , Si and Mn. Aluminum and its amalgams can be given states of pipe, poles, tubes, wires, plates or foils and in this manner discover utilizes as a part of pressing, utensil making, plane, construction, and transportation industry.
-
The utilization of aluminum and its compounds for local objects is decreased impressively due to their lethal nature.
Facts: Boron Cage Structure
Fig. 5: Clusters of 40 boron atoms form a molecular cage similar to the carbon buckyball.
Researchers have shown that clusters of 40 boron atoms form a molecular cage similar to the carbon buckyball. This is the first experimental evidence that such a boron cage structure exists.
Frequently Asked Questions (FAQs)
Q1. What group is Boron in?
Sol. Group 13
Q2. Which group does Aluminium belong to?
Sol. Group 13
Q3. What is Boron and what is it used for?
Sol. Boron is a chemical element with symbol B and atomic number 5 in group 13. Immaculate boron is a dark amorphous powder. Amorphous boron is utilized as a rocket fuel igniter and in pyrotechnic flares. It gives the flares particular green shading.
Q4. Is Boron radioactive?
Sol. Three radioactive isotopes of boron are known too. A radioactive isotope is one that breaks off and gives some type of radiation. None of the radioactive isotopes of boron have any vital industrial uses. Two normally happening isotopes of boron exist: boron-10 and boron-11. Boron-10 is the isotope with high neutron-retaining inclinations.
Watch this Video for more reference
More Readings