Amorphous and Crystalline Solids
Table of contents |
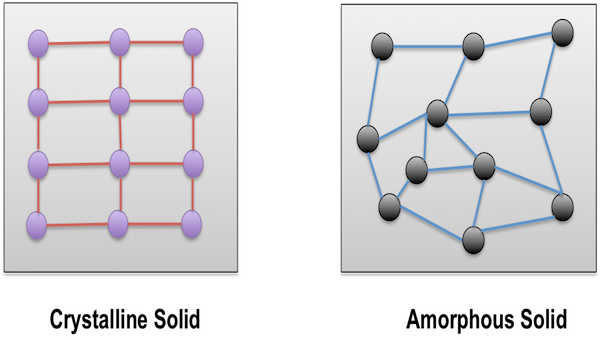
Types of Solids
On the basis of the arrangement of constituent particles, the solids are classified into two categories, namely:
Image 1: Arrangement of Atoms
Amorphous Solids
The solids in which the constituent particles of matter are arranged in a random manner are called amorphous solids. It is a non-crystalline solid with no proper arrangement of atoms in the solid lattice. In other words, we can define amorphous solids as materials which don’t have certain organized arrangement of atoms and molecules. Most solids are amorphous in nature and are utilized in many sectors as well. One of the most common examples of amorphous solids is glass, which is used widely in the manufacturing sector.
Characteristics of Amorphous Solids
An Amorphous Solid depicts following properties, which are as follows:
-
The constituent particles of matter inside solid are arranged in a random manner, that is, the position of atoms and molecules is not fixed and varies from one solid to another
-
Amorphous Solids don’t have definite shape or geometry due to random arrangement of atoms and molecules inside the solid lattice
-
Short-range order is found in amorphous solids
-
Amorphous Solids are also called Pseudo-solids or Supercooled Liquids because they don’t form crystalline structure and has the ability to flow
-
The nature of amorphous solids is isotropic in nature that is, the properties measured in all directions come out to be same, example refractive index of amorphous solids is same
-
Amorphous solids don’t show sharp melting point, this is because of irregular packing of amorphous solids
-
When we cut an amorphous solid, we find the broken constituent particles to be irregular in shape and geometry
-
Amorphous solids are unsymmetrical in nature, due to irregular packing of atoms and molecules inside the solid lattice
-
Amorphous solids don’t have fixed heat of fusion because of absence of sharp melting point
Examples: Plastics, Glass, Rubber, Metallic Glass, Polymers, Gel etc.
There are many applications of amorphous solids, some of them are:
-
The glass is widely used in packaging (food jars, cosmetics box, and soft-drink bottles), making tableware (utensils), in the construction of buildings ( windows, lighting, and shelves) etc.
-
Rubber is mainly used in manufacturing of tires, footwear, ropes, camp cloth and as a raw material for several industries
-
Use of polymer can be seen in manufacturing of pipes, medicines and as a raw ingredient for many factories
-
Amorphous silicon is considered as the best photovoltaic material to convert sunlight into electricity
Crystalline Solids
The solids in which the constituent particles of matter are arranged and organized in a specific manner are called Crystalline Solids. These solids contain crystals in their structure and each crystal has definite geometry. Adding further, as crystalline solids have low potential energy, they are the most stable form of solids. Almost all solids fall in the category of crystalline solids including metallic elements (iron, silver, and copper) and non-metallic elements (Phosphorus, Sulphur, and iodine). Also several compounds like sodium chloride, zinc sulphide and naphthalene build crystalline solids.
Image 2: Example of Crystalline Solids
Characteristics of Crystalline Solids
The main characteristics of crystalline solids are mentioned as below:
-
Crystalline solids show regular structure and have definite geometrical shape
-
The sharp freezing point is found in crystalline solids. This is because the distance between same atoms/molecules or ions is same and remains constant, unlikely from amorphous solids
-
The heat of fusion is definite and fixed as the regularity in crystal lattice remains same and is ideal
-
Crystalline Solids are also known as True Solids as they don’t tend to flow like pseudo solids
-
When we cut a crystal solids with a knife, we obtain a flat and smooth surface
-
The nature of crystalline solid is anisotropic; that is, the properties turn out to be different in different direction
-
Crystalline solids depict both long range and short range order
Examples: Quartz, Calcite, Sugar, Mica, Diamonds etc.
Image 3: Lattice Structure of Crystalline Solids
Uses of Crystalline Solids
There are many applications of crystalline solids, some are:
-
Diamond is the most decent example of crystalline solids and is widely used in making beautiful jewelry items
-
Quartz is extensively used in manufacturing of watches and clocks
-
Many crystalline solids are used as a raw material in many industries
Image 4: Structure of NaCl
Crystalline Solids are further classified into four categories on the basis of intermolecular interactions between molecules, they are:
-
Molecular Solids
-
Covalent or Network Solids
-
Ionic Solids
-
Metallic Solids
Image 5: Shape of Crystalline and Amorphous Solids
Molecular Solids
In molecular solids, the constituent particles are molecules. Molecular solids are generally insulators and are soft in nature. The density of molecular solids is quite low. Based on nature of molecules molecular solids are further classified into three forms:
-
Non-polar Molecular Solids
-
Polar Molecular Solids
-
Hydrogen-bonded Molecular Solids
Covalent Solids
In covalent solids, the constituent atoms of molecules held together by strong covalent bonds. They form giant structures and are generally hard in nature. They are also poor conductors of electricity except graphite, which is a good conductor of electricity.
Ionic Solids
The constituent particles in ionic solids are cations and anions. There is strong electrostatic force of attraction between particles. Ionic solids are hard and brittle. They have very high melting point. Ionic solids are poor conductor of electricity in solid state, but in dissolved state, they act as good conductor of electricity. For Example, NaCl is a good conductor of electricity in dissolved state.
Metallic Solids
The constituent particles in metallic solids are metal atoms and valence electrons. Metallic Solids have high melting and boiling point. They are also good conductor of electricity due to presence of valence electrons. For Example: Copper, Gold etc.
Difference between Crystalline and Amorphous Solids
The difference between crystalline and amorphous solids can be laid out in the table below:
Characteristic |
Crystalline Solids |
Amorphous Solids |
|
Melting Point |
Melt at fixed temperature |
Melts steadily over range of temperatures |
|
Arrangement of Constituent Particles |
Regular |
Irregular |
|
Shape |
Regular and Definite Shape |
Irregular Shape in Nature |
|
Cleavage |
When cut, two smooth and plain pieces are obtained |
When cut, two surfaces of irregular shape is obtained |
|
Heat of Fusion |
Definite |
Indefinite |
|
Anisotropy |
Anisotropic |
Isotropic |
|
Nature |
True Solids |
Pseudo Solids |
Watch this Video for more reference
More Readings