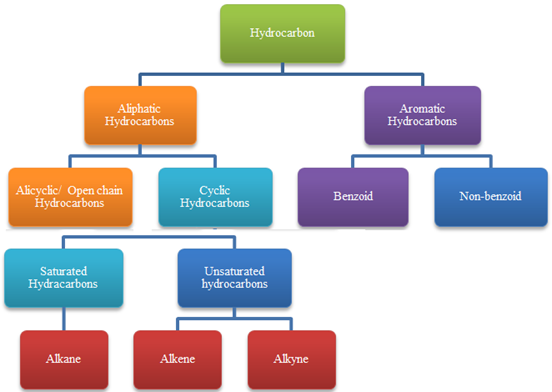
Hydrocarbons
-
Compounds of carbon and hydrogen.
-
Classification of Hydrocarbons:
Alkane
-
Open chain saturated hydrocarbon with general formula (CnH2n+2).
-
All the C atoms are single bonded i.e. sp3 hybridised.
Conformations of Alkane
-
Conformations are the different arrangement of atoms that can be converted into one another by rotation about single bonds.
-
Eclipsed Conformation: H atoms on two adjacent carbon atoms are closest to each other i.e. dihedral angle is 0.

-
Staggered Conformation: H atoms on two adjacent carbon atoms are farthest to each other i.e. dihedral angle is 60.


Preparation of Alkanes:
-
Reduction of Alkyl Halides:
RX + Zn: + H+ → RH + Zn2+ + X-
4RX + LiAlH4 → 4RH + LiX + AlX3 (X≠ F)
RX + (n - C4H9)3 SnH → R-H + (n - C4H9)3 SnX
-
Grignard Reagent:
?
-
Hydrogenation of Alkenes:

-
Wurtz Reaction:
2RX + 2Na → R-R + 2NaX
2Na + 2CH3CH2CH2Cl → CH3CH2CH2CH2-CH2CH3 + 2NaCl
-
Corey House Reaction:
![]()
-
Decarboxylation of a mixture of the sodium salt of a carboxylic acid:
RCOONa +NaOH(CaO) → RH + Na2CO3
-
Kolbe's electrolytic method:
2 RCOOK + 2H2O → R-R + 2CO2 + H2+ 2KOH
Chemical Properties of Alkane
-
Direct Halogenation
RH + X2→ RX + HX
Order of Reactivity of X2: F2 > Cl2 > Br2; I2 does not react
?a. Initiation Step
Cl-Cl  2Cl.
2Cl.
b. Propagation Step
H3C-H +Cl. → H3C. + H-Cl
H3C. + Cl-Cl → H3C-Cl +Cl.
c. Termination Step
Cl. + Cl. →Cl-Cl
H3C. + H3C. → H3C-CH3
Cl. + H3C. → Cl-CH3
-
Nitration
Nitration of alkane is made by heating vapours of alkanes and HNO3 at about 400oC to give nitroalkanes.
¨This is also known as vapour phase nitration.

-
Combustion:
?Alkanes burn readily with non luminous flame in presence of air or oxygen to give CO2 & water along with evolution of heat.
C2H6 + 7O2 → CO2 +6H2O + heat
-
Aromatization
?¨Alkanes having six to 10 carbon atoms are converted into benzene and its homologues at high pressure and temperature in presence of catalyst.

-
Oxidization of 30 alkane:?
Tertiary alkanes are oxidized to tertiary alcoholsby KMnO4
R3CH + KMnO4 → R3COH
Alkene (olefins)
-
Open chain, Unsaturated hydrocarbons with general formula (CnH2n).
-
At least one >c=c< (double bond) group i.e. sp2 hybridisation, is present throughout the chain.
-
Allene: alkene molecule in which at least one C has double bonds with each of the adjacent carbon i.e. -c=c=c- group.
-
Isomeric with saturated cycloalkanes.

Geometric Isomers:

Z is used if the higher - priority substituents on each C are on the same side of the double bond.letter E is used if they are on opposite sides

Heats of Hydrogenation: Heat of hydrogenation increases with increase in stability of alkene.

Order of heat of hydrogenation: 1-Butene> cis-2-Butene > trans-2-Butene
Order of stability: 1-Butene> cis-2-Butene > trans-2-Butene
Preparation of Alkenes:
1. Cracking of petroleum: ![]()
2. Dehydrohalogenation of alkyl halides: RCH2CH2X + alc.KOH → RCH = CH2
3. Dehydration of Alcohols :
Saytzeff Rule: In dehydration and dehydrohalogenation the preferential order for removal ofan H is 3° > 2° > 1°

4. Reduction of alkynes:

Chemical Properties:
1. Electrophilic Polar Addition Reactions
|
Reagent |
Product |
||
|
Name |
Structure |
Name |
Structure |
|
Halogens (Cl2, Br2 only) |
X:X |
Ethylene dihalide |
CH2XCH2X |
|
Hydrohalic acids |
H:X |
Ethyl halide |
CH3CH2X |
|
Hypohalous acids |
X:OH |
Ethylene halohydrin |
CH2XCH2OH |
|
Sulfuric acid (cold) |
H:OSO2OH |
Ethyl bisulfate |
CH3CH2OSO3H |
|
Water (dil. H3O+) |
H:OH |
Ethyl alcohol |
CH3CH2OH |
|
Borane
|
H2B:H |
Ethyl borane |
(CH3CH2BH2) → (CH3CH2)3B |
|
Peroxyformic acid
|
H:O-OCH=O (HCO3H) |
Ethylene glycol |
CH2OHCH2OH |
2. Addition of Hydrogen Halides to Alkenes: Markovnikov’s Addition:
R - CH = CH2 + HBr → R – CHBr – CH3
Mechanism:
R - CH = CH2 + HBr → R – CH+ - CH3 +Br-
R – CH+ - CH3 + Br- → R – CHBr - CH3
Anit- Markovnikov’s Addition (Peroxide Effect):
R - CH = CH2 + HBr + (C6H5CO)2O2 → R – CHBr – CH3
Mechanism
Initiation:
R - O - O - R → 2RO.
RO. + HBr → Br. + ROH
Propagation
CH3CH = CH2 + Br. → CH3·CH - CH2Br
CH3·CHCH2Br + HBr→ CH3CH2CH2Br + Br.
Termination:
2RO. → R - O - O - R
Br. + Br.→Br2
3. Addition of Water to Alkenes: Acid Catalyzed Hydration:
|
Reagent |
Product |
||
|
Name |
Structure |
Name |
Structure |
|
Halogens (Cl2, Br2 only) |
X:X |
Ethylene dihalide |
CH2XCH2X |
|
Hydrohalic acids |
H:X |
Ethyl halide |
CH3CH2X |
|
Hypohalous acids |
X:OH |
Ethylene halohydrin |
CH2XCH2OH |
|
Sulfuric acid (cold) |
H:OSO2OH |
Ethyl bisulfate |
CH3CH2OSO3H |
|
Water (dil. H3O+) |
H:OH |
Ethyl alcohol |
CH3CH2OH |
|
Borane
|
H2B:H |
Ethyl borane |
(CH3CH2BH2)®(CH3CH2)3B |
|
Peroxyformic acid
|
H:O - OCH = O (HCO3H) |
Ethylene glycol |
CH2OHCH2OH |

4. Oxymercuration-Demercuration:

Examples:

5. Hydroboration-Oxidation:

Examples:

6. Halogen Addition in Non-polar Solvent:

7. Halogen Addition in Aqueous Medium:

8. Syn – Hydroxylation: Formation of di-oles.

9. Ozonolysis of Alkenes:

Alkyne
-
Saturated open chain hydrocarbon with general formula (CnH2n-2).
-
At least one -c≡c- (triple bond) group i.e. sp hybridisation, is present throughout the chain.
-
Physical properties of alkynes are similar to those of the corresponding alkenes
Preparation
1. Dehydrohalogenation of vic-Dihalides or gem-Dihalides

2. Dehalogenation of vic-Tetrahalogen Compounds

3. Alkyl Substitution in Acetylene; Acidity of º C-H

4. From Calcium Carbide:
CaC2 +2H2O → Ca(OH)2+ C2H2
5. Kolbe’s Electrolysis:

Chemical Properties
1. Hydrogenation: RC ≡ CCH2CH3 + 2H2 → CH3CH2CH2CH2CH3
2. Hydro-halogenation:
Markovnikov addition: RC≡CH +HBr → RCBr=CH2 +HBr→ RCBr2-CH3
Anti-markovnikov addition: RC≡CH +HBr +peroxide → RCH=CHBr

Aromatic Hydrocarbons:
For being aromatic a hydrocarbon should
-
be a cyclic compounds.
-
have planarity in geometry.
-
have complete delocalization of electrons over ring.
-
follow Huckel Rule i.e. number of ?? electrons in ring = (4n+2). :

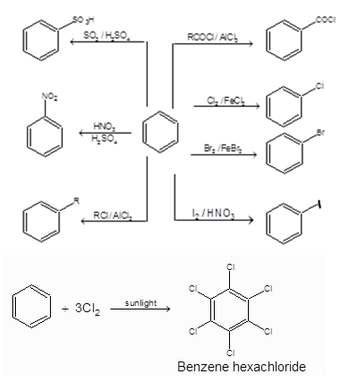
Benzene (C6H6)
1. Structure:

2. Chemical Reactions of Benzene:
Anti-aromatic Hydrocarbons:
Highly unstable compounds.
Number of π electrons in ring = 4n.
Example:

You Might Like to Refer:
cbse website for class 9 |CBSE online classes | CBSE online class 12