Proteins and Amino Acids
Table of Content |
Protein
 The word protein was coined by Berzelius in 1838 and was used by G. J. Mulder first time 1840.
The word protein was coined by Berzelius in 1838 and was used by G. J. Mulder first time 1840.
15% of protoplasm is made up of protein.
Average proteins contain 16% nitrogen, 50–55% carbon, oxygen 20–24%, hydrogen 7% and sulphur 0.3 – 0.5%. Iron, phosphorous, copper, calcium, and iodine are also present in small quantity.
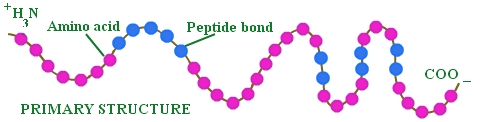
Structure of Proteins
It is due to different rearrangement of amino acids. When carboxyl group (-COOH) of one amino acid bonded with amino group (– NH2) of another amino acid the bond is called peptide bond. A peptide may be dipeptide, tripeptide and polypeptide. The simplest protein is Insulin. According to Sanger (1953) insulin consists of 51 amino acids. A protein can have up to four level of conformation.
(i) Primary structure : The primary structure is the covalent connections of a protein. It refers to linear sequence, number and nature of amino acids bonded together with peptide bonds only. e.g. ribonuclease, insulin, haemoglobin, etc.
(ii) Secondary structure : The folding of a linear polypeptide chain into specific coiled structure (α - helix) is called secondary structure and if it is with intermolecular hydrogen bonds the structure is known as ß -pleated sheet. α -helical structure is found in protein of fur, keratin of hair claws, and feathers. ß -pleated structure is found in silk fibres.
(iii) Tertiary structure : The arrangement and interconnection of proteins into specific loops and bends is called tertiary structure of proteins. It is stabilized by hydrogen bond, ionic bond, hydrophobic bond and disulphide bonds. It is found in myoglobin (globular proteins).
(iv) Quaternary structure : It is shown by protein containing more than one peptide chain. The protein consists of identical units. It is known as homologous quaternary structure e.g. lactic dehydrogenase. If the units are dissimilar, it is called as heterogeneous quaternary structure e.g. hemoglobin which consists of two α -chains and two ß -chains.
Classification of Proteins
Proteins are classified on the basis of their shape, constitution and function.
On the basis of shape
 (i) Fibrous protein/Scleroprotein : Insoluble in water. Animal protein resistant to proteolytic enzyme is spirally coiled thread like structure form fibres. e.g. collagen (in connective tissue), actin and myosin, keratin in hairs, claws, feathers, etc.
(i) Fibrous protein/Scleroprotein : Insoluble in water. Animal protein resistant to proteolytic enzyme is spirally coiled thread like structure form fibres. e.g. collagen (in connective tissue), actin and myosin, keratin in hairs, claws, feathers, etc.
(ii) Globular proteins : Soluble in water. Polypeptides coiled about themselves to form oval or spherical molecules e.g. albumin insulin hormones like ACTH, oxytosin, etc.
On the basis of constituents
(i) Simple proteins : The proteins which are made up of amino acids only. e.g. albumins, globulins, prolamins, glutelins, histones, etc.
(ii) Conjugated proteins : These are complex proteins combined with characterstic non–amino acid substance called as prosthetic group. These are of following types :–
(a) Nucleoproteins : Combination of protein and nucleic acids, found in chromosomes and ribosomes. e.g. deoxyribonucleoproteins, ribonucleoproteins, etc.
(b) Mucoproteins : These are combined with large amount (more than 4%) of carbohydrates e.g. mucin.
(c) Glycoproteins : In this, carbohydrate content is less (about 2 – 3%) e.g. immunoglobulins or antibiotics.
(d) Chromoproteins : These are compounds of protein and coloured pigments. e.g. haemoglobin, cytochrome, etc.
(e) Lipoproteins : These are water soluble proteins and contain lipids. e.g. cholesterol and serum lipoproteins.
(f) Metalloprotein : These are metal binding proteins, AB1–globin known as transferring is capable of combining with iron, zinc and copper e.g. chlorophyll.
(g) Phosphoprotein : They composed of protein and phosphate e.g. casein (milk) and vitellin (egg).
(iii) Derived proteins : When proteins are hydrolysed by acids, alkalies or enzymes, the degredation products obtained from them are called derived proteins. On the basis of progressive cleavage, derived proteins are classified as primary proteoses, secondary proteoses, peptones, polypeptides, amino acids, etc.
On the basis of nature of molecules
(i) Acidic proteins : They exist as anion and include acidic amino acids. e.g. blood groups.
(ii) Basic proteins : They exist as cations and rich in basic amino acids e.g. lysine, arginine etc.
Function of Proteins
(i) Proteins occur as food reserves as glutelin, globulin casein in milk.
(ii) Proteins are coagulated in solutions, alkaline to the isoelectric pH by positive ions such as Zn2+, Cd2+, Hg2+ etc. Casein – pH 4.6, cyt. C – 9.8, resum globulin 5.4, pepsin 2.7, lysozyme 11.0 etc.
(iii) Proteins are the most diverse molecule on the earth.
(iv) Proteins work as hormone as insulin and glucagon.
(v) Antibiotics as gramicidin, tyrocidin and penicillin are peptides.
(vi) They are structural component of cell.
(vii) They are biological buffers.
(viii) Monellin is the sweetest substance obtained from African berry (2000 time sweeter than sucrose).
(ix) Proteins helps in defence, movement activity of muscles, visual pigments receptor molecules, etc.
(x) Natural silk is a polyamide and artificial silk is a polysaccharide. Nitrogen is the basic constituent.
Amino Acids
Amino acids are normal components of cell proteins (called amino acid). They are 20 in number specified in genetic code and universal in viruses, prokaryotes and eukaryotes. Otherwise amino acids may be termed rare amino acids, which take part in protein synthesis e.g. hydroxyproline and non- protein amino acids do not take part in protein synthesis e.g. Ornithin, citrullin, gama-aminobutyric acid (GABA) a neurotransmitter, etc.
Structure and Composition
Amino acids are basic units of protein and made up of C, H, O, N and sometimes S. Amino acids are organic acids with a carboxyl group (–COOH) and one amino group (-NH2) on the a -carbon atom. Carboxyl group attributes acidic properties and amino group gives basic ones. In solution, they serve as buffers and help to maintain pH. General formula is R-CHNH2.COOH.
Amino acids are amphoteric or bipolar ions or Zueitter ions. Amino acids link with each other by peptide bond and long chains are called polypeptide chains.
Classification
Based on R-group of amino acids.
(a) Simple amino acids : These have no functional group in the side chain. e.g. glycine, alanine , leucine, valine etc.
(b) Hydroxy amino acids : They have alcohol group in side chain. e.g. threonine, serine, etc.
(c) Sulphur containing amino acids : They have sulphur atom in side chain. e.g. methionine, cystenine.
(d) Basic amino acids : They have basic group (-NH2) in side chain. e.g. lysine, arginine.
(e) Acidic amino acids : They have carboxyl group in side chain. e.g. aspartic acid, glutamic acid.
(f) Acid amide amino acids : These are the derivatives of acidic amino acids. In this group, one of the carboxyl group has been converted to amide (-CONH2). e.g. asparagine, glutamine.
(g) Heterocyclic amino acids : These are the amino acids in which the side chain includes a ring involving at least one atom other than carbon. e.g. tryptophan, histidine.
(h) Aromatic amino acids : They have aromatic group (benzene ring) in the side chain. e.g. phenylalanine, tyrosine, etc.
On the basis of requirements : On the basis of the synthesis amino acids in body and their requirement, they are categorized as :–
(a) Essential amino acids : These are not synthesized in body hence to be provided in diet e.g. valine, leucine, isoleucine, theronine ,lysine, etc.
(b) Semi-essential amino acids : Synthesized partially in the body but not at the rate to meet the requirement of individual. e.g., arginine and histidine.
(c) Non-essential amino acids : These amino acids are derived from carbon skeleton of lipids and carbohydrate metabolism. In humans there are 12 non- essential amino acids e.g. alanine, aspartic acid, cysteine, glutamic acid etc. Proline and hydroxyproline have, NH (imino group) instead of NH2 hence are called imino acids. Tyrosine can be converted into hormone thyroxine and adrenaline and skin pigment melanin. Glycine is necessary for production of heme. Tryptophan is the precursor of vitamin nicotinamide and auxins. If amino group is removed from amino acid it can form glucose and if COOH group is removed, it forms amines e.g. histamine.
Note:
Functions of Amino Acids
(a) Amino acids are building blocks of proteins and enzymes.
(b) By glycogenolysis, they form glucose.
(c) Hormones like adrenaline and thyroxine are formed with the help of tyrosine.
(d) Antibiotics often contain non-protein amino acids.
(e) They are precursour of many substances.


Q.1: Peptide bond is formed between two amino acids through [Kerala 2001]
(a) Addition of water (b) Loss of water
(c) Decarboxylation (d) Deamination
Q.2: A basic amino acid is [DPMT 2001]
(a) Leucine (b) Methionine
(c) Aspartic acid (d) Lysine
Q.3: Most abundant organic compound on earth is [CBSE PMT 2001]
(a) Cellulose (b) Protein
(c) Lipids (d) Steriods
Q.4: Most common monosaccharides found in nucleus are [AIIMS 1996; CMC 2002]
(a) Trioses (b) Tetroses
(c) Pentoses (d) Hexoses
Q.5: Hydrolysis of nucleic acid yields [AMU 1988]
(a) Only sugar (b) Phosphoric acid only
(c) NItrogenous base only (d) All of the above
Q.6: Nucleic acids were discovered by [MP PMT 1999]
(a) Watson and Crick (b) Khorana
(c) Wilkins (d) Miescher
Q.7: A nucleoside differs from a nucleotide in not having [MP PMT 1998]
(a) Phosphate (b) Sugar
(c) Phosphate and sugar (d) Nitrogen base
Q.8: DNA was first discovered by [CPMT 1979; BHU 1985]
(a) Beadle and Tatum (b) Watson and Crick
(c) Friedrich Miescher (d) Kornberg
![]()

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
|
d |
b |
b |
|
|
Q.5 |
Q.6 |
Q.7 |
Q.8 |
|
c |
c |
b |
b |
Related Resources
-
Click here to refer the Useful Books of Biology for NEET (AIPMT)
-
Click here for study material on Cell – the unit of life
To read more, Buy study materials of Biomolecules comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Biology here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free