November 8, 2013
New Delhi: We all knew that warm water freezes faster than water at a lower temperature ever since Aristotle. It also has a name – Mpemba effect. But now, a team of researchers from Nanyang Technological University of Singapore have discovered ‘why’ it happens. They suggest that the secret is in the way the small amount of energy is stored in the stretched hydrogen bonds between the water molecules.
It was a riddle that had been puzzling scientists for thousands of years.
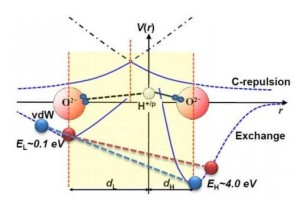
Those who have even basic knowledge of covalent bonds understand that a water molecule has one oxygen atom and two hydrogen atoms that are held together by sharing their electrons. Hydrogen atoms are also attracted to oxygen atoms of other water molecules nearby – forming hydrogen bonds. On the other hand, water molecules repel each other as a whole.
The team at NTU noted that these repellent forces get stronger as the water gets warmer and distances between water molecules increase. They force the hydrogen bonds to become stretched storing more energy in them than the hydrogen bonds of cooler water.
Thus, when warm and cool water are exposed to freezing temperatures, hydrogen bonds in warmer water have more energy to release, leading to faster cooling and freezing of water.
However, this is just a theory proposed by the research team and it is still to be seen whether it can be proven true in due time.
This post was written by Aditya Singhal, managing director of askIITians.