The Back Bencher’s Tip to learn the Periodic Table
Mnemonics are easy-to-remember lines or phrases one can use to memorize things that are difficult to learn. In this article, you will find Hindi mnemonics – one each for one group – to learn the Periodic Table
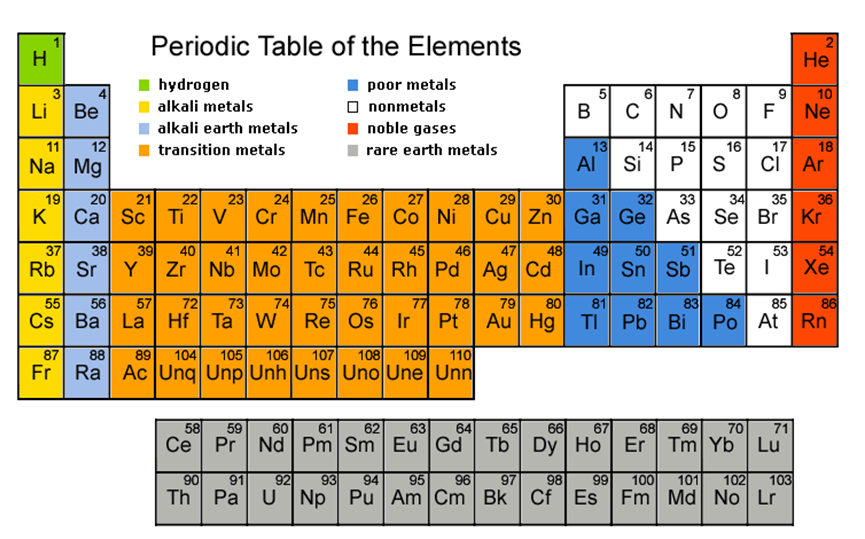
The Periodic Table provides the names, atomic numbers, symbols and atomic weights of known elements. It serves as a great tool for solving chemistry problems.
A periodic table is divided into groups (columns), where elements with each group behave similarly while bonding with other elements; and periods (rows), where elements in one period have same number of electron shells.
Here are some fun, interesting and naughty mnemonics in Hindi used by the backbenchers to memorize elements along each group or period:
Key To Reading These Mnemonics Or Hindi Sentence:
• These sentences contain letters denoting symbols of elements in the same order as they occur in a group or period.
• The symbols have been highlighted as bold letters in the sentence. However at the places where the complete symbol could not be included in the sentence, the first letters have been strung together and the second letter is shown in brackets. While reading the sentence you don’t have to read the letters in bracket. Just keep them in mind.
• At some places, phonetics have been used to denote a symbols such as ‘c’ could be replaced by ‘k’,’g’ with ‘j’, ‘I’ with ‘ea’ and ‘o’ with ‘u’, to make the sentence easier to remember.
S-Block Elements
Consisting of the first two groups, S-block elements have quite similar physical and chemical properties. The valence electrons of the elements in this block occupy s-orbitals.
Group 1 is known as alkali metals. It includes Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Ru), Caesium (Cs), and Francium (Fr).
Mnemonic for Group 1: LiNa Ki Ruby Cse Friendship hai.
Group 2 is known as alkaline earth metals. It includes Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Br), and Radium (Ra).
Mnemonic for Group 2: Beta Mange Car Scooter Baap rone se Raazi
P-Block Elements
Consisting of last six groups of the periodic table (Groups 13 to 18), P-block elements have their valence electrons occupying p-orbitals.  This block consists of non-metals, semi-metals and poor metals.
Group 13 is known as Boron group or the group of Icosagens or Triels. It includes Boron (B), Aluminium (Al), Gallium (Ga), Indium (In), and Thallium (Tl).
Mnemonic for Group 13: B A G I T.
Group 14 is known as Carbon group or the group of Crystallogens, Tetragens or Tetrels. It includes Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn), and Lead (Pb).
Mnemonic for Group 14: Chemistry Sir Gives Sanki Problems.
Group 15 is known as the group of Pnictogens or Nitrogen group. It includes Nitrogen (N), Phosphorus (P), Arsenic (As), Antimony (Sb), and Bismuth (Bi).
Mnemonic for Group 15: Nahi Pasand Aise Sab Bhai.
Group 16 is known as the group of Chalcogens or Oxygen group. It includes Oxygen (O), Sulphur (S), Selenium (Se), Tellurium (Te), and the radioactive element Polonium (Po).
Mnemonic for Group 16: Oh! Style Se Tel Polish.
Group 17 is known as the group of Halogens. It includes Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (At).
Mnemonic for Group 17: Fir Call kar Bahaar AayI Aunty.
Group 18 is known as the group of Noble gases, excluding Helium. Normally, they are all odorless and colorless gases with very low chemical reactivity. The group includes Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and the radioactive Radon (Rn).
Mnemonic for Group 18: He Never Arrived; Kara Xero Run pe out.
D-Block Elements
D-Block elements consist of element groups 3 to 12 that correspond to the filling of the d-orbital subshell of the second outermost shell. Groups 3 to 11 are also known as transitional metals. Group 12 elements, which have its d subshell completely filled, are also known as post-transition elements.
D-block elements and F-block elements show considerable similarities across the periods too.
We can memorize these elements across the periods:
Period 4 elements are quite stable and many of them are very common in earth’s crust or core or both. D-block elements it includes are Scandium (Sc), Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese (Mn), Iron (Fe), Cobalt (Co), Nickel (Ni), Copper (Cu) and Zinc (Zn).
Mnemonic for Period 4: Science Ti(ea)cher Vineeta Criplani Man Fenko (FeCo) Ni Kyun(Cu) Zaan hai?
Read as: Science Teacher Vineeta Kriplani manfenko ni kyun zaan hai?
Period 5 elements are known to fill their 5s shell first, then 4d shells and then 5p shells, with rhodium being the exception. The elements of this period show many exceptions to Maledung rule. D-block elements it includes are Yttrium (Y), Zirconium (Zr), Niobium (Nb), Molybdenum (Mo), Technetium (Tc), Ruthenium (Ru), Rhodium (Rh), Pd (Palladium), Silver (Ag) and Cadmium (Cd).
Mnemonic for Period 5: Yeh Zarra Nabi bana Mohabaat mein T(c)eri, R(u)o R(h)o P(d)ukarogi Aaj(g) ise Chandni
Read as: Yeh Zarra Nabi bana Mohabbat mein Teri, Ro Ro Pukarogi Aaj ise Chandni
Period 6 includes the lanthanides or rare earths. Some of these transition metals are very valuable such as gold. D-block elements it includes are Lutetium (Lu), Hafnium (Hf), Tantalum (Ta), Tungsten (W), Rhenium (Re), Osmium (Os), Iridium (Ir), Platinum (Pt), Gold (Au) and Mercury (Hg).
Mnemonic for Period 6: L(u)a HafTa Warna Reh Us(Os) Irritating Popat ke saath Aur Hoj(g)a pagal.
Read as: La Hafta Warna Reh Us Irritating Popat ke saath Aur Hoja pagal.
Period 7 contains the radioactive elements only. It includes actinides which include the heaviest naturally occurring element Californium. All other elements are synthesized artificially. D-block elements
it includes are Actinium (Ac), Rutherfordium (Rf), Dubnium (Db), Seaborgium (Sg), Bohrium (Bh), Hassium (Hs), Meitnerium (Mt), and Darmstadtium (Ds).
Mnemonic for Period 7: Ak(c)ele R(f) D(b) S(g)harma ki B(h)ook mein H(s)ain Maths ke Difficult sawaal.
Read as: Akele R D Sharma ki Book mein Hain Maths ke Difficult sawaal.
F-Block Elements
F-block elements have their valence electrons in f-orbitals. They are also known as inner transition elements. They can be divided into Lanthanides (also known as rare earth elements) and Actinides that are highly reactive to halogens and chalcogens like lanthanides but they react more easily.
Lanthanides include Cerium (Ce), Praseodymium (Pr), Neodymium (Nd), Promethium (Pm), Samarium (Sm), Europium (Eu), Gadolinium (Gd), Terbium (Tb), Dysprosium (Dy), Holmium (Ho), Erbium (Er), Thulium (Tm), Ytterbium (Yb) and Lutetium (Lu).
We can learn all these in three parts:
1.     Cerium (Ce), Praseodymium (Pr), Neodymium (Nd), Promethium (Pm), and Samarium (Sm)
Mnemonic for Lanthanides Part 1: Celina aur Priety Ne dande se Pammy aur Simmy ko mara.
2.     Europium (Eu), Gadolinium (Gd), Terbium (Tb), Dysprosium (Dy), and Holmium (Ho)
Mnemonic for Lanthanides Part 2: Europe G(d)aya to TB(b) aur Di(y)arrohoea Ho gaya.
Read as: Europe Gaya to TB aur Diarrohoea Ho gaya.
3.     Erbium (Er), Thulium (Tm), Ytterbium (Yb) and Lutetium (Lu)
Mnemonic for Lanthanides Part 3: E re, dekh Tamatar Yellow aur bLue hain.
Actinides include these f-block elements – Thorium (Th), Protactinium (Pa), Uranium (U), Neptunium (Np), Plutonium (Pu), Americium (Am), Curium (Cm), Berkelium (Bk), Fermium (Fm), Mendelevium (Md), Nobelium (No), and Lawrencium (Lr).
We can learn all these in three parts too:
1.Thorium (Th), Protactinium (Pa), Uranium (U), and Neptunium (Np)
Mnemonic for Actinides Part 1: Thode Pehelwan Unse Niptengey.
2.Plutonium (Pu), Americium (Am), Curium (Cm), Berkelium (Bk)
Mnemonic for Actinides Part 2: Purane Aam K(C)am Bikenge.
Read as: Purane Aam Kam Bikenge.
3. Fermium (Fm), Mendelevium (Md), Nobelium (No), and Lawrencium (Lr)
Mnemonic for Actinides Part 3: Itni Family aMdani mein No Ladki rajee.
To help us bring you breaking news and Informative Articles please subscribe to our blog! This post was written by Aditya Singhal, managing director of askIITians

thanks alot……….
i m in 10th and i really needed it!!!
thank u once again!!
follow me on twitter- @parth3399
10 th mein iss ki zaroorat nahi
Very good
great job ! but you have missed out and shuffled some elements while making mnemonics….eg Cf and Es(Californium and einsteinium)in actinide series have been missed and period 7 elements have been shuffled …
à ¤ªà ¥€à ¤°à ¤¿à ¤¯à ¤¾à ¤¡à ¤¿à ¤• à ¤Ÿà ¥‡à ¤¬à ¤²- à ¤®à ¥‡à ¤®à ¥‹à ¤°à ¥€ à ¤—à ¥Âà ¤°à ¥ (MemoryGuru)
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
1 à ¤¸à ¥‡ 10 à ¤¤à ¤¤à ¥Âà ¤µ
à ¤¹à ¤¾à ¤ˆà ¤¡à ¥Âà ¤°à ¥‹ à ¤¹à ¥‡à ¤²à ¥€ à ¤²à ¥€à ¤¥à ¥€ à ¤¬à ¥‡à ¤°à ¥€
à ¤¬à ¥‹à ¤° à ¤•à ¤¾à ¤°à ¥Âà ¤¬à ¤¨ à ¤¨à ¤¿à ¤¤ à ¤°à ¥‹à ¤¤à ¥€ à ¤¹à ¥ˆ
à ¤…à ¤•à ¥Âà ¤¸ à ¥žà ¥‚à ¤² à ¤•à ¥‹ à ¤¨à ¤¯à ¤¨ à ¤°à ¤–à ¥‡ à ¤¯à ¥‡ à ¤¨à ¤•à ¤šà ¥Âà ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
1 – à ¤¹à ¤¾à ¤‡à ¤¡à ¥Âà ¤°à ¥‹à ¤œà ¤¨ – H – Hydrogen 2 – à ¤¹à ¤¿à ¤²à ¤¿à ¤¯à ¤® – He – Helium 3 – à ¤²à ¤¿à ¤¥à ¤¿à ¤¯à ¤® – Li – Lithium 4 – à ¤¬à ¥‡à ¤°à ¤¿à ¤²à ¤¿à ¤¯à ¤® – Be – Beryllium 5 – à ¤¬à ¥‹à ¤°à ¥‰à ¤¨à ¥ â€“ B – Boron 6 – à ¤•à ¤¾à ¤°à ¥Âà ¤¬à ¤¨ – C – Carbon 7 – à ¤¨à ¤¾à ¤‡à ¤Ÿà ¥Âà ¤°à ¥‹à ¤œà ¤¨ – N – Nitrogen 8 – à ¤‘à ¤•à ¥Âà ¤¸à ¥€à ¤œà ¤¨ – O – Oxygen 9 – à ¤«à ¥Âà ¤²à ¥‹à ¤°à ¥€à ¤¨ – F – Fluorine 10 – à ¤¨à ¤¿à ¤¯à ¥‹à ¤¨ – Ne – Neon
11à ¤¸à ¥‡20à ¤¤à ¤¤à ¥Âà ¤µ
à ¤¸à ¥‹à ¤¡à ¤¾ à ¤®à ¤— à ¤®à ¥‡ à ¤…à ¤² à ¤¸à ¥€à ¤²à ¥€ à ¤²à ¤¾à ¤¯à ¥€
à ¥žà ¥Âà ¤¸à ¥žà ¥Âà ¤¸ à ¤—à ¤‚à ¤§à ¤• à ¤•à ¥Âà ¤²à ¥‹à ¤°à ¥€à ¤¨ à ¤®à ¤¿à ¤²à ¤¾à ¤¯à ¥€
à ¤®à ¤¿à ¤² à ¤—à ¤¯à ¤¾ à ¤…à ¤°à ¤—à ¥‹ à ¤®à ¥‡à ¤‚ à ¤ªà ¥‹à ¤Ÿà ¤¾à ¤¶ à ¤†à ¤šà ¥‚à ¤¨à ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ …
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
11 – à ¤¸à ¥‹à ¤¡à ¤¿à ¤¯à ¤® – Na  Sodium (à ¤²à ¥ˆà ¤Ÿà ¤¿à ¤¨ à ¤¶à ¤¬à ¥Âà ¤¦ ‘Natrium’ à ¤¸à ¥‡) 12 – à ¤®à ¥ˆà ¤—à ¥Âà ¤¨à ¥‡à ¤¶à ¤¿à ¤¯à ¤® – Mg – Magnesium 13 – à ¤Âà ¤²à ¥Âà ¤¯à ¥Âà ¤®à ¤¿à ¤¨à ¤¿à ¤¯à ¤® – Al – Aluminium 14 – à ¤¸à ¤¿à ¤²à ¤¿à ¤•à ¥‰à ¤¨ – Si – Silicon 15 – à ¤«à ¤¾à ¤¸à ¥Âà ¤«à ¥‹à ¤°à ¤¸ – P – Phosphorus 16 – à ¤—à ¤¨à ¥Âà ¤§à ¤• – S – Sulfur 17 – à ¤•à ¥Âà ¤²à ¥‹à ¤°à ¥€à ¤¨ – Cl – Chlorine 18 – à ¤‘à ¤°à ¥Âà ¤—à ¤¨ – Ar – Argon 19 – à ¤ªà ¥‹à ¤Ÿà ¥ˆà ¤¶à ¤¿à ¤¯à ¤® – K  Potassium (à ¤œà ¤°à ¥Âà ¤®à ¤¨ à ¤Âà ¤¾à ¤·à ¤¾ à ¤•à ¥‡ à ¤¶à ¤¬à ¥Âà ¤¦ ‘Kalium’ à ¤¸à ¥‡) 20 – à ¤•à ¥ˆà ¤²à ¥Âà ¤¶à ¤¿à ¤¯à ¤® – Ca  Calcium
21à ¤¸à ¥‡30 à ¤¤à ¤¤à ¥Âà ¤µ
à ¤¸à ¥Âà ¤•à ¤¨à ¥Âà ¤¦ à ¤¨à ¤•à ¥Âà ¤·à ¤¤à ¥Âà ¤° à ¤®à ¥‡à ¤‚ à ¤Ÿà ¤¾à ¤ˆà ¤Ÿà ¥‡à ¤¨à ¤¿à ¤• à ¤ªà ¤° à ¤¬à ¥ˆà ¤ à ¤µà ¤¨à ¥Âà ¤¦à ¤¨à ¤¾
à ¤•à ¥Âà ¤°à ¥‹à ¤® à ¤ªà ¤¹à ¤¿à ¤¨à ¤•à ¤° à ¤®à ¤¨à ¤—à ¤¨à ¥‡à ¤¶ à ¤¸à ¥‡ à ¤²à ¥‹à ¤¹à ¤¾ à ¤²à ¥‡à ¤•à ¤°
à ¤•à ¥‹à ¤¬à ¤°à ¤¾ à ¤¨à ¤¿à ¤•à ¤² à ¤¤à ¤¾à ¤‚à ¤¬à ¥‡ à ¤®à ¥‡à ¤‚ à ¤•à ¤° à ¤—à ¤¯à ¤¾ à ¤¸à ¤¿à ¤‚à ¤•
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ …
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
21 – à ¤¸à ¥Âà ¤•à ¤¾à ¤£à ¥Âà ¤¡à ¤¿à ¤¯à ¤® – Sc – Scandium 22 – à ¤Ÿà ¤¾à ¤‡à ¤Ÿà ¤¾à ¤¨à ¤¿à ¤¯à ¤® – Ti – Titanium 23 – à ¤µà ¤¨à ¥‡à ¤¡à ¤¿à ¤¯à ¤® – V – Vanadium 24 – à ¤•à ¥Âà ¤°à ¥‹à ¤®à ¤¿à ¤¯à ¤® – Cr – Chromium 25 – à ¤®à ¥ˆà ¤‚à ¤—à ¤¨à ¥€à ¤œ – Mn – Manganese 26 – à ¤²à ¥‹à ¤¹à ¤¾ – Fe  Iron (à ¤²à ¥ˆà ¤Ÿà ¤¿à ¤¨ à ¤¶à ¤¬à ¥Âà ¤¦ ‘Ferrum’ à ¤¸à ¥‡) 27 – à ¤•à ¥‹à ¤¬à ¤¾à ¤²à ¥Âà ¤Ÿ – Co – Cobalt 28 – à ¤¨à ¤¿à ¤•à ¥‡à ¤² – Ni – Nickel 29 – à ¤¤à ¤¾à ¤®à ¥Âà ¤° – Cu  Copper (à ¤²à ¥ˆà ¤Ÿà ¤¿à ¤¨ à ¤¶à ¤¬à ¥Âà ¤¦ ‘Cuprum’ à ¤¸à ¥‡) 30 – à ¤œà ¤¸à ¥Âà ¤¤à ¤¾ – Zn – Zinc
31à ¤¸à ¥‡40 à ¤¤à ¤¤à ¥Âà ¤µ
à ¤—à ¤²à ¥€ à ¤®à ¥‡à ¤‚ à ¤œà ¤°à ¥Âà ¤®à ¤¨ à ¤¶à ¤¨à ¥Âà ¤–à ¤¿à ¤¯à ¤¾ à ¤¬à ¥‡à ¤‚à ¤šà ¥‡
à ¤¬à ¥Âà ¤°à ¤¹à ¥Âà ¤® à ¤•à ¥ƒà ¤ªà ¤¾à ¤£ à ¤°à ¥‚à ¤¬à ¥€ à ¤•à ¥‹ à ¤¦à ¥‡à ¤–à ¥‡
à ¤¸à ¥Âà ¤Ÿà ¥Âà ¤°à ¤¾à ¤¨à ¥Âà ¤— à ¤¯à ¤¾à ¤¤à ¥Âà ¤°à ¥€ à ¥›à ¤¿à ¤°à ¤•à ¥‹à ¤¨à ¥€ à ¤®à ¥‡à ¤‚ à ¤•à ¤°à ¥‡ à ¤—à ¥œà ¤¬à ¥œà ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
31 – à ¤—à ¥ˆà ¤²à ¤¿à ¤¯à ¤® – Ga – Gallium 32 – à ¤œà ¤°à ¥Âà ¤®à ¥‡à ¤¨à ¤¿à ¤¯à ¤® – Ge – Germanium 33 – à ¤†à ¤°à ¥Âà ¤¸à ¥‡à ¤¨à ¤¿à ¤• – As – Arsenic 34 – à ¤¸à ¥‡à ¤²à ¥‡à ¤¨à ¤¿à ¤¯à ¤® – Se – Selenium 35 – à ¤¬à ¥Âà ¤°à ¥‹à ¤®à ¤¿à ¤¨ – Br – Bromine 36 – à ¤•à ¥Âà ¤°à ¤¿à ¤ªà ¥Âà ¤Ÿà ¤¨ – Kr – Krypton 37 – à ¤°à ¥Âà ¤¬à ¤¿à ¤¡à ¤¿à ¤¯à ¤® – Rb – Rubidium 38 – à ¤¸à ¥Âà ¤Ÿà ¥Âà ¤°à ¥‹à ¤¨à ¥Âà ¤¸à ¤¿à ¤¯à ¤® – Sr – Strontium 39 – à ¤‡à ¤¤à ¥Âà ¤°à ¤¿à ¤¯à ¤® – Y – Yttrium 40 – à ¤œà ¤°à ¥Âà ¤•à ¥‹à ¤¨à ¤¿à ¤¯à ¤® – Zr  Zirconium
41à ¤¸à ¥‡50 à ¤¤à ¤¤à ¥Âà ¤µ
à ¤¨à ¤¾à ¤¯à ¤¾à ¤¬ à ¤®à ¤¾à ¤²à ¥€ à ¤Ÿà ¥‡à ¤•à ¥‚ à ¤°à ¥Âà ¤ à ¤—à ¤¯à ¤¾
à ¤°à ¥‹à ¤¡ à ¤ªà ¤° à ¤ªà ¤²à ¥œà ¥‡ à ¤®à ¥‡à ¤‚ à ¤šà ¤¾à ¤¨à ¥Âà ¤¦à ¥€ à ¤²à ¥‡à ¤•à ¤° à ¤¬à ¥ˆà ¤ à ¤—à ¤¯à ¤¾
à ¤•à ¤¦à ¤®à ¥Âà ¤¬ à ¤¡à ¤¾à ¤² à ¤ªà ¤° à ¤‡à ¤¨à ¥Âà ¤¦à ¥‚ à ¤Ÿà ¥€à ¤¨à ¥‡ à ¤®à ¥‡à ¤‚ à ¤²à ¥‡ à ¤—à ¤¯à ¥€ à ¤Âà ¤°
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
41 – à ¤¨à ¤¾à ¤¯à ¥‹à ¤¬à ¤¿à ¤¯à ¤® – Nb – Niobium 42 – à ¤®à ¥‹à ¤²à ¤¿à ¤¬à ¥Âà ¤¡à ¥‡à ¤¨à ¤® – Mo – Molybdenum 43 – à ¤Ÿà ¥‡à ¤•à ¥Âà ¤¨à ¤¿à ¤¶à ¤¿à ¤¯à ¤® – Tc  Technetium 44 – à ¤°à ¥Âà ¤¥à ¥‡à ¤¨à ¤¿à ¤¯à ¤® – Ru – Ruthenium 45 – à ¤°à ¥‹à ¤¡à ¤¿à ¤¯à ¤® – Rh – Rhodium 46 – à ¤ªà ¤²à ¤¾à ¤¡à ¤¿à ¤¯à ¤® – Pd – Palladium 47 – à ¤šà ¤¾à ¤Âà ¤¦à ¥€ – Ag  Silver (à ¤²à ¥ˆà ¤Ÿà ¤¿à ¤¨ à ¤¶à ¤¬à ¥Âà ¤¦ ‘Argentum’ à ¤¸à ¥‡) 48 – à ¤•à ¤¾à ¤¡à ¤®à ¤¿à ¤¯à ¤® – Cd – Cadmium 49 – à ¤‡à ¤£à ¥Âà ¤¡à ¤¿à ¤¯à ¤® – In – Indium 50 – à ¤¤à ¥Âà ¤°à ¤ªà ¥ â€“ Sn  Tin (à ¤²à ¥ˆà ¤Ÿà ¤¿à ¤¨ à ¤¶à ¤¬à ¥Âà ¤¦ ‘Stannum’ à ¤¸à ¥‡)
51 à ¤¸à ¥‡ 60 à ¤¤à ¤¤à ¥Âà ¤µ
à ¤…à ¤¨à ¥Âà ¤Ÿà ¥€ à ¤¬à ¥‡à ¤²à ¥€ à ¤†à ¤¯à ¥‹à ¤¡à ¥€à ¤¨ à ¤•à ¤¾ à ¤œà ¤¿à ¤¨à ¥Âà ¤¨à ¤¾à ¤¨ à ¤²à ¤¾à ¤¯à ¤¾
à ¤¶à ¥€à ¤¶à ¥€ à ¤¬à ¥ˆà ¤°à ¥€ à ¤²à ¤ à ¥Âà ¤ à ¤¾ à ¤¶à ¥‡à ¤°à ¥€
à ¤ªà ¥Âà ¤°à ¤¸à ¤¾à ¤¦ à ¤¨à ¤µà ¥‹à ¤¦à ¤¯ à ¤¤à ¤¾à ¤¶ à ¤•à ¥‡ à ¤ªà ¤¤à ¥Âà ¤¤à ¥‡ à ¤–à ¤¾ à ¤—à ¤¯à ¤¾
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
51 – à ¤Âà ¤¨à ¥Âà ¤Ÿà ¤¿à ¤®à ¥‹à ¤¨à ¥€ – Sb  Antimony (à ¤²à ¥ˆà ¤Ÿà ¤¿à ¤¨ à ¤¶à ¤¬à ¥Âà ¤¦ ‘ Stibium’ à ¤¸à ¥‡) 52 – à ¤Ÿà ¥‡à ¤²à ¥Âà ¤°à ¤¿à ¤¯à ¤® – Te – Tellurium 53 – à ¤†à ¤¯à ¥‹à ¤¡à ¤¿à ¤¨ – I – Iodine 54 – à ¤œà ¤¼à ¥‡à ¤¨à ¤¾à ¤¨ – Xe – Xenon 55 – à ¤¸à ¥€à ¤œà ¤¼à ¤¿à ¤¯à ¤® – Cs – Caesium 56 – à ¤¬à ¥‡à ¤°à ¤¿à ¤¯à ¤® – Ba – Barium 57 – à ¤²à ¤¾à ¤žà ¥Âà ¤¥à ¤¨à ¤® – La – Lanthanum 58 – à ¤¸à ¥‡à ¤°à ¤¿à ¤¯à ¤® – Ce – Cerium 59 – à ¤ªà ¥Âà ¤°à ¤¾à ¤¸à ¤¿à ¤¯à ¥‹à ¤¡à ¤¾à ¤‡à ¤®à ¤¿à ¤¯à ¤® – Pr – Praseodymium 60 – à ¤¨à ¤¿à ¤¯à ¥‹à ¤¡à ¤¾à ¤‡à ¤®à ¤¿à ¤¯à ¤® – Nd – Neodymium
61à ¤¸à ¥‡ 70 à ¤¤à ¤¤à ¥Âà ¤µ
à ¤ªà ¥Âà ¤°à ¥‹à ¤®à ¥‹à ¤¶à ¤¨ à ¤•à ¥€ à ¤¸à ¤®à ¤°à ¥€ à ¤®à ¥‡à ¤‚ à ¤¯à ¥‚à ¤°à ¥‹ à ¤—à ¤¾à ¥œà ¥‹
à ¤¤à ¤°à ¤¬à ¥€ à ¤¡à ¤¿à ¤¸à ¥Âà ¤ªà ¥‹à ¤œà ¤² à ¤•à ¤° à ¤¦à ¥‹ à ¤¹à ¥‹à ¤²à ¥€ à ¤®à ¥‡à ¤‚
à ¤…à ¤°à ¤¬à ¥€ à ¤¥à ¥Âà ¤²-2 à ¤‡à ¤Ÿà ¤°-2 à ¤•à ¤° à ¤•à ¤°à ¥‡ à ¤—à ¥œà ¤¬à ¥œà ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
61 â€†à ¤ªà ¥Âà ¤°à ¥‹à ¤®à ¥‡à ¤¥à ¤¿à ¤¯à ¤®  Pm  Promethium 62 â€†à ¤¸à ¥ˆà ¤®à ¤°à ¤¿à ¤¯à ¤®  Sm  Samarium 63 â€†à ¤¯à ¥Âà ¤°à ¥‹à ¤ªà ¤¿à ¤¯à ¤®  Eu  Europium 64 â€†à ¤—à ¥Âà ¤¯à ¤¾à ¤¡à ¥‹à ¤²à ¤¿à ¤¨à ¤¿à ¤¯à ¤®  Gd  Gadolinium 65 â€†à ¤Ÿà ¤°à ¥Âà ¤¬à ¤¿à ¤¯à ¤®  Tb  Terbium 66 â€†à ¤¡à ¤¿à ¤¸à ¥Âà ¤ªà ¥Âà ¤°à ¥‹à ¤¸à ¤¿à ¤¯à ¤®  Dy  Dysprosium 67 â€†à ¤¹à ¥‹à ¤²à ¥Âà ¤®à ¤¿à ¤¯à ¤®  Ho  Holmium 68 â€†à ¤…à ¤°à ¥Âà ¤¬à ¤¿à ¤¯à ¤®  Er  Erbium 69 â€†à ¤¥à ¥Âà ¤²à ¤¿à ¤¯à ¤®  Tm  Thulium 70 â€†à ¤¯à ¤¿à ¤Ÿà ¥Âà ¤Ÿà ¤°à ¤¬à ¤¿à ¤¯à ¤®  Yb  Ytterbium
71à ¤¸à ¥‡80 à ¤¤à ¤¤à ¥Âà ¤µ
à ¤²à ¥‚à ¤Ÿà ¥‡ à ¤¹à ¤¾à ¥žà ¥‡ à ¤Ÿà ¥Âà ¤‚à ¤Ÿà ¤¾ à ¤Ÿà ¤‚à ¤—à ¤¾ à ¤°à ¤¾à ¤¨à ¥€ à ¤“à ¤¸ à ¤¨à ¤¹à ¤¾à ¤Â
à ¤‡à ¤°à ¥€à ¤¡à ¥€ à ¤®à ¥‡à ¤‚ à ¤ªà ¤²à ¤Ÿà ¥‡ à ¤¸à ¥‹à ¤¨à ¤¾ à ¤ªà ¤¾à ¤°à ¤¾ à ¤Šà ¤ªà ¤° à ¤œà ¤¾à ¤Â
à ¤…à ¤¸à ¥Âà ¤¸à ¥€ à ¤•à ¤¾ à ¤…à ¤¬ à ¤¬à ¥‚à ¥Âà ¤¾ à ¤¹à ¥‹à ¤•à ¤° à ¤¹à ¤® à ¤•à ¥Âà ¤¯à ¤¾ à ¤¹à ¥ˆ à ¤ªà ¥œà ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
71 – à ¤²à ¥Âà ¤Ÿà ¥‡à ¤Ÿà ¤¿à ¤¯à ¤® – Lu – Lutetium 72 – à ¤¹à ¤¾à ¤«à ¥Âà ¤¨à ¤¿à ¤¯à ¤® – Hf – Hafnium 73 – à ¤Ÿà ¤¾à ¤£à ¥Âà ¤Ÿà ¤²à ¤® – Ta – Tantalum 74 – à ¤Ÿà ¤‚à ¤—à ¥Âà ¤¸à ¥Âà ¤Ÿà ¤¨ – W  Tungsten (à ¤œà ¤°à ¥Âà ¤®à ¤¨ à ¤Âà ¤¾à ¤·à ¤¾ à ¤•à ¥‡ à ¤¶à ¤¬à ¥Âà ¤¦ ‘Wolfram’ à ¤¸à ¥‡)) 75 – à ¤°à ¥‡à ¤¨à ¤¿à ¤¯à ¤® – Re – Rhenium 76 – à ¤…à ¤¸à ¥Âà ¤®à ¤¿à ¤¯à ¤® – Os – Osmium 77 – à ¤‡à ¤°à ¤¿à ¤¡à ¤¿à ¤¯à ¤® – Ir – Iridium 78 – à ¤ªà ¥Âà ¤²à ¤¾à ¤Ÿà ¤¿à ¤¨à ¤® – Pt – Platinum 79 – à ¤¸à ¥‹à ¤¨à ¤¾ – Au  Gold (à ¤²à ¥ˆà ¤Ÿà ¤¿à ¤¨ à ¤¶à ¤¬à ¥Âà ¤¦ ‘Aurum’ à ¤¸à ¥‡) 80 – à ¤ªà ¤¾à ¤°à ¤¾ – Hg  Mercury (à ¤²à ¥ˆà ¤Ÿà ¤¿à ¤¨ à ¤¶à ¤¬à ¥Âà ¤¦ ‘Hydragyrum’ à ¤¸à ¥‡)
81à ¤¸à ¥‡ 90 à ¤¤à ¤¤à ¥Âà ¤µ
à ¤¥à ¤¾à ¤²à ¥€ à ¤¶à ¥€à ¤¶à ¤¾ à ¤¬à ¤¿à ¤¸à ¥Âà ¤•à ¥Âà ¤Ÿ à ¤ªà ¥‹à ¤²à ¥‹
à ¤…à ¤·à ¥Âà ¤Ÿà ¥Âà ¤§à ¤¾à ¤¤à ¥ à ¤•à ¥€ à ¤°à ¤¾à ¤¡à ¥‹ à ¤ªà ¤¹à ¤¨à ¥‹
à ¥žà ¥Âà ¤°à ¤¾à ¤¨à ¥Âà ¤¸ à ¤°à ¥‡à ¤¡à ¤¿à ¤¯à ¤® à ¤Âà ¤•à ¥Âà ¤Ÿà ¤¿à ¤‚à ¤— à ¤¥à ¥‹à ¥œà ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
81 – à ¤¥à ¥ˆà ¤²à ¤¿à ¤¯à ¤® – Tl – Thallium 82 – à ¤¸à ¥€à ¤¸à ¤¾ – Pb  Lead (à ¤²à ¥ˆà ¤Ÿà ¤¿à ¤¨ à ¤¶à ¤¬à ¥Âà ¤¦ ‘Plumbum’ à ¤¸à ¥‡) 83 – à ¤¬à ¤¿à ¤¸à ¥Âà ¤®à ¤¥ – Bi – Bismuth 84 – à ¤ªà ¥‹à ¤²à ¥‹à ¤¨à ¤¿à ¤¯à ¤® – Po – Polonium 85 – à ¤Âà ¤¸à ¥Âà ¤Ÿà ¤¾à ¤Ÿà ¤¿à ¤¨ – At – Astatine 86 – à ¤°à ¥‡à ¤¡à ¤¨ – Rn – Radon 87 – à ¤«à ¥Âà ¤°à ¤¾à ¤¨à ¥Âà ¤¸à ¤¿à ¤¯à ¤® – Fr – Francium 88 – à ¤°à ¥‡à ¤¡à ¤¿à ¤¯à ¤® – Ra – Radium 89 – à ¤Âà ¤•à ¥Âà ¤Ÿà ¤¿à ¤¨à ¤¿à ¤¯à ¤® – Ac – Actinium 90 – à ¤¥à ¥‹à ¤°à ¤¿à ¤¯à ¤® – Th – Thorium
91à ¤¸à ¥‡100à ¤¤à ¤¤à ¥Âà ¤µ
à ¤ªà ¤°à ¥‡ à ¤¤à ¤¾à ¤•à ¤¤à ¥€ à ¤¯à ¥‚à ¤°à ¥‡à ¤¨à ¤¸ à ¤¨à ¥‡à ¤ªà ¥Âà ¤šà ¥‚à ¤¨ à ¤ªà ¥Âà ¤²à ¥‚à ¤Ÿà ¥‹
à ¤…à ¤®à ¤°à ¥€à ¤•à ¤¨ à ¤•à ¥Âà ¤¯à ¥‚à ¤°à ¥€ à ¤ªà ¤¹à ¤¿à ¤¨à ¥‡ à ¤¬à ¤°à ¤•à ¤¾
à ¤•à ¥‡à ¤²à ¥žà ¥‹à ¤°à ¥Âà ¤¨à ¤¿à ¤¯à ¤¾ à ¤®à ¥‡à ¤‚ à ¤†à ¤‡à ¤‚à ¤¸à ¤Ÿà ¥€à ¤¨ à ¤¸à ¥Âà ¤¨à ¤¾à ¤¯à ¥‡ à ¥žà ¤°à ¤®à ¤¾à ¤¨à ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
91 – à ¤ªà ¥Âà ¤°à ¥‹à ¤Ÿà ¥ˆà ¤•à ¥Âà ¤Ÿà ¥€à ¤¨à ¤¿à ¤¯à ¤® – Pa – Protactinium 92 – à ¤¯à ¥Âà ¤°à ¥‡à ¤¨à ¤¿à ¤¯à ¤® – U – Uranium 93 – à ¤¨à ¥‡à ¤ªà ¥Âà ¤Ÿà ¥Âà ¤¯à ¥‚à ¤¨à ¤¿à ¤¯à ¤® – Np – Neptunium 94 – à ¤ªà ¥Âà ¤²à ¥‚à ¤Ÿà ¥‹à ¤¨à ¤¿à ¤¯à ¤® – Pu – Plutonium 95 – à ¤…à ¤®à ¥‡à ¤°à ¤¿à ¤¶à ¤¿à ¤¯à ¤® – Am – Americium 96 – à ¤•à ¥Âà ¤¯à ¥‚à ¤°à ¤¿à ¤¯à ¤® – Cm – Curium 97 – à ¤¬à ¤°à ¥Âà ¤•à ¥‡à ¤²à ¤¿à ¤¯à ¤® – Bk – Berkelium 98 – à ¤•à ¥ˆà ¤²à ¥€à ¤«à ¥‹à ¤°à ¥Âà ¤¨à ¤¿à ¤¯à ¤® – Cf – Californium 99 – à ¤†à ¤‡à ¤¨à ¥Âà ¤¸à ¥Âà ¤Ÿà ¤¾à ¤‡à ¤¨à ¤¿à ¤¯à ¤® – Es – Einsteinium 100 – à ¤«à ¤°à ¥Âà ¤®à ¤¿à ¤¯à ¤® – Fm – Fermium
101à ¤¸à ¥‡ 112 à ¤¤à ¤¤à ¥Âà ¤µ
à ¤®à ¤‚à ¤¡à ¤² à ¤œà ¥€à ¤¤à ¤¾ à ¤¨à ¥‹à ¤¬à ¤² à ¤²à ¤¾à ¤°à ¥‡à ¤‚à ¤¸ à ¤¶à ¤¹à ¤° à ¤®à ¥‡à ¤‚
à ¤¡à ¥Âà ¤¬à ¤¨à ¥€ à ¤¶à ¥€à ¤¬à ¥‹ à ¤¬à ¥‹à ¤¹à ¤°à ¥€ à ¤¬à ¥ˆà ¤ à ¥€ à ¤¹à ¤¾à ¤¸à ¤¿à ¤® à ¤®à ¥€à ¤Ÿà ¤° à ¤¦à ¥‡à ¤–à ¥‡à ¥¤
à ¤¡à ¤°à ¤®à ¤¸à ¥Âà ¤¤ à ¤°à ¥‹ à ¤‡à ¤‚à ¤¤à ¤œà ¤¾à ¤° à ¤®à ¥‡à ¤‚ à ¤•à ¤¾à ¤ªà ¤°à ¥Âà ¤¨à ¤¿à ¤•à ¤¸ à ¤›à ¥œà ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
101 – à ¤®à ¥‡à ¤£à ¥Âà ¤¡à ¥‡à ¤²à ¥€à ¤µà ¤¿à ¤¯à ¤® – Md – Mendelevium 102 – à ¤¨à ¥‹à ¤¬à ¥‡à ¤²à ¤¿à ¤¯à ¤® – No – Nobelium 103 – à ¤²à ¥‰à ¤°à ¥‡à ¤‚à ¤¶à ¤¿à ¤¯à ¤® – Lr – Lawrencium 104 – à ¤°à ¥Âà ¤¥à ¤°à ¤«à ¥‹à ¤°à ¥Âà ¤¡à ¤¿à ¤¯à ¤® – Rf – Rutherfordium 105 – à ¤¡à ¤¬à ¥Âà ¤¨à ¤¿à ¤¯à ¤® – Db – Dubnium 106 – à ¤¸à ¥€à ¤¬à ¥‹à ¤°à ¥Âà ¤—à ¤¿à ¤¯à ¤® – Sg – Seaborgium 107 – à ¤¬à ¥‹à ¤°à ¤¿à ¤¯à ¤® – Bh – Bohrium 108 – à ¤¹à ¤¸à ¤¿à ¤¯à ¤® – Hs – Hassium 109 – à ¤®à ¥‡à ¤‡à ¤Ÿà ¥Âà ¤¨à ¥‡à ¤°à ¤¿à ¤¯à ¤® – Mt – Meitnerium 110 – à ¤¡à ¤¾à ¤°à ¥Âà ¤®à ¥Âà ¤¸à ¥Âà ¤Ÿà ¥‡à ¤¡à ¤¶à ¤¿à ¤¯à ¤® – Ds – Darmstadtium 111 – à ¤°à ¥‰à ¤¨à ¥Âà ¤Ÿà ¤œà ¥ˆà ¤¨à ¤¿à ¤¯à ¤® – Rg – Roentgenium 112 – à ¤‰à ¤¨à ¤‰à ¤¨à ¤¬à ¤¿à ¤¯à ¤® – Uub – Copernicium
113à ¤¸à ¥‡ 118 à ¤¤à ¤¤à ¥Âà ¤µ
à ¤…à ¤¨ à ¤…à ¤¨ à ¤•à ¤°à ¤¤à ¥€ à ¤¤à ¤¿à ¤°à ¤¿à ¤¯à ¤® à ¤•à ¥Âà ¤µà ¤¾à ¤¡à ¥‹
à ¤ªà ¥ˆà ¤¨à ¥Âà ¤Ÿà ¥€ à ¤¹à ¥‡à ¤•à ¥Âà ¤¸à ¥€ à ¤²à ¤¾à ¤¯à ¥‡
à ¤¸à ¥‡à ¤ªà ¥Âà ¤Ÿà ¥€ à ¤†à ¤ à ¤˜à ¤¾à ¤˜à ¤°à ¤¾ à ¤ªà ¤¹à ¤¿à ¤¨à ¥‡ à ¤°à ¤¹à ¥‡ à ¤–à ¥œà ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€ à ¤†à ¤µà ¤°à ¥Âà ¤¤ à ¤¸à ¤¾à ¤°à ¤£à ¥€
113 – à ¤‰à ¤¨à ¤‰à ¤¨à ¤Ÿà ¥Âà ¤°à ¤¿à ¤¯à ¤® – Uut – Ununtrium 114 – à ¤‰à ¤¨à ¤‰à ¤¨à ¤•à ¥Âà ¤µà ¤¾à ¤¡à ¥ƒà ¤¯à ¤® – Uuq – Ununquadium 115 – à ¤‰à ¤¨à ¤‰à ¤¨à ¤ªà ¥ˆà ¤¨à ¥Âà ¤¶à ¤¿à ¤¯à ¤® – Uup – Ununpentium 116 – à ¤‰à ¤¨à ¤‰à ¤¨à ¤¹à ¥ˆà ¤•à ¥Âà ¤·à ¤¿à ¤¯à ¤® – Uuh – Ununhexium 117 – à ¤‰à ¤¨à ¤‰à ¤¨à ¤¸à ¥ˆà ¤ªà ¥Âà ¤•à ¥Âà ¤·à ¤¿à ¤¯à ¤® – Uus – Ununseptium 118 – à ¤‰à ¤¨à ¤‰à ¤¨à ¤‘à ¤•à ¥Âà ¤·à ¤¿à ¤¯à ¤® – Uuo  Ununoctium
1st group he loko na kro rab ca fr ayad
2nd group bache mere kaise sare bathe rahte
3rd period na mang al si pure sapne kar le apne
these are my techniques i made them to memorise these
Give some more
i read in 10+1 thanx for ur rattaficative lines
thnx bro it was really helpful & nice…………
I liked but i don’t indersted in some point so that i am confuse
I liked but i don’t indersted in some point so that i am confuse please can you help me in periodic table
very good. A very amusing way to remember boring long periodic table
Nyc i really want this thnxxxx alot ……………
Really helpful.
really gud nd hlpfl……mks lrning enjyble.;):)
Helped a lot to memorise it over-night…… Really helpfull
some better tips:’
13th grp :- Baingan Allu Gajar In Thali
15 th grp:- Nana Patekar Aur Sab Bimar
and the best
16th grp :-O S Se Te Po (read as uss se te po) matlab copy from him
mast thanks’
O S Se Te Po o sanam sexy teri pose
I love your memonics
ya very usefull
Awesome
it really helped me
thanxxx,it helped a lot
Glad to see “Physics for Backbenchers” book is now out! The
book is written to drive away the fear of learning hard concepts of
physics and math and promote science education in India. Check this out
on flipkart and amazon. By the same author, “The Speed of Time” book is a
bestseller number 1 in Science and Technology category.
In Group 1, please correct Rubidium’s symbol to Rb (from Ru). Ru is Ruthenium.
From Ramanand
1st group: HaLiNa Ki Rab Cse Fariyaad
2nd group: BeMag Ca Sir BaRa
when did your heart go missing?
IITIANS ARE ALWAYS ROCKSSSSSSSSSSSSSS…..THIS PERIODIC TABLE SHOWS THAT.VERY GOOD PERIODIC TABLE.
THANKYOU
“Barium (Br)” barium has the symbol Ba, not Br
excellent
some more tips
Lanthanoids :- LAst CEll PaR Not Disturb ka ProMo Sun(m) kar EUhi GoD ko Than(M)x DiYa wHOle yEaR TuM Yuhi(b) LUta
Cene par nadiya prem samayi eu gadgad tab dil hua engineer tum yuhi lute
some more tips
Gr. 13:-BAl GAngadhar Indian Tilaik
Gr. 14- Car Se(I) Gaye Sndy ko Pub
Gr 15- No Problem AS SBI
Gr 16- Ore Sanam Se Tel Poche
15th group: Nana patekar asia me sabse bidwan
thanks really helpful u r great
for first 9 atomic numbers
Hello HEllo LIsten B(e) B C News Of France
capital letters give the symbol
13th group- Batta Aloo Gobhi IN Thela
30 to 40 atomic no.-gana gate aye sabhi bahana karke rulaye sabhi yehi zindigi
Period3 Na Mago Ala se pepsi soda cola aur(more)
4- science teacher vinita croplani mann Firoj ko (co) Niraj ne Cuch zyada Gali de ge aur usSE bhi Bura kha
SaChin TIendulkar Very CRazy MaN For (fe) Cricket(CO) New Zealand (Ni) calcutta (cu) Zimbabwe (Zn)
tip for reactivity series
katrina- K
Na ne
car-Ca
mangi-Mg
alto-Al
zen-Zn
ferrari-Fe
parvin babi-Pb
kyon (nothing)
ho gayi-(Hg)
angry-(Ag)
aunty-(Au)
reactivity as per decreasing order
Better still
(k) katrina
(na) ne
(ca) car
(mg) mangi
(al) alto
(zn) zen
(fe) ferrari
(pb) phir bhi kyuon
(hg/mercury) mili
(ag/silver) silver
(au/gold) audi
Katrina ne car mangi alto, zen, ferrari phur bhi kyon mili silver audi.
Really nice…
osmm..
à ¤²à ¤¿à ¤• à ¤¬à ¥‡à ¤¸à ¤° à ¤•à ¥‡à ¤¨à ¤¾ à ¤®à ¤‚à ¤—à ¤² à ¤®à ¤‚à ¤œà ¤¨ à ¤•à ¤° à ¥žà ¥€à ¤•à ¤¡ à ¤¹ à ¤•à ¥‰à ¤ªà ¤° à ¤šà ¤¾à ¤‚à ¤¦à ¥€ à ¤¸à ¥‹à ¤¨à ¤¾ ( I Br Cl F)
Li K Ba Sr Ca Na Mg Al Mn Zn Cr Fe Cd H Cu Ag Au (I Br Cl F)
Helpful thanks…!
ya its really good
15th grp Na PaAS SBBhI
Not at all helpful…. Bhai kha se soch k banaya hai tune ye….. Small time waste kar diya
i cant understand the sentences over here
Which one?
Tip for remembering reactivity series:
Please (Potassium)
Stop (Sodium)
Calling (Calcium)
My (magnesium)
Angry (Aluminium)
Zebra (Zinc)
Ian (iron)
The (Tin)
Lovely (Lead)
*Happy* (*Hydrogen*)
Coffin (Copper)
Sighs (Silver)
Granny (Gold)
Platypus (Platinum)
Good job….but please make the mnemonics of f block elements a bit easy…..do it as soon as possible I’ll check the site again
BEST EVER
(d block 4th period elements)
sc- sachin
ti-tendulkar is
v-very
cr-crucial
mn-man
fe-fir
co-kyu
ni-naa
cu-kuch
zn-zane
Hey
Hi
Listen
B
B
C
News
On
Friday
Night
Naa
Mard
Alsi
Please
Salee
Kalti
Le
Aur
Khuja
Ke
Ca Ban JA
Science
Leke
Tv mat dekhna
Crore
Me
Kamana
Helped me a lot great job guys
14 C,SI,GE,SN,PB (kahe shiv ji suno parvati)
1A group { H, Li ,Na, K Rb, Se, Fr} (He Lila na kar Rab Se Fariyad)
2A group {be,mg,ca,sr,ba,ra) Beta mange car scooter baap raji)
13 group- (B,Al,Ga,In,Tl) {bhindi Aaloo gajar in thaila }
14 group {c,si,ge ,sn,pb) -{ kahe shiv ji suno parvati }
15 group- (N,p,As, Sb,Bi) -{Nana patekar aishwarya sab bindas)
16 group-(O,S,Se,Te,Po)- { O samrat Sena Tere pass)
17 group -F,cl,Br,I,At ( fir kal bahar aayi aunty)
18 group -(he,ne,Ar,Kr, Xe,Rn ) ( Hilo nahi agar karana hai X-ray)
Actinoids :
Thari Pant U Napu Ki America Ka CM Bankok Me Coffee Pikar Eswariya Ki Film K.M.D Ke Nokar Se Lade
Thari = Th
Pant = Pa
U = U
Napu = Np and Pu
America = Am
CM = Cm
Bankok = Bk
Coffee = Cf
Eswariya = Es
Film = Fm
KMD = Md
Nokar= No
Lade = Lr
Wow…. its a kudos extoll psych n sheer funny to learn pt..
Einstenium and californium are missing :/
lina kabr se farrar alkaline metals
21 to 30
Sachin tendulkar very crazy man for cricket never care zimbabme
Sc ti v cr mn fe co ni cu zn
Group 15-Nati pota asha Sab bemar
Group 16-os se Teri pooja
(Now my favourite)
Group 18-hero Ne Aruna kumari ka xenon radon kardia
In the 2nd series of f block, between berkelium n fermium, californium n einstenium are missin..
Reactivity series
Po so ka mal jitl by co mesi
Thank you for sharing your valuable article. This is very useful and helpful for many entrance exams.
Only Possible because of this blog
To learn periodic table
In 30 mins
Thank you !!!
Loved this blog
Actinides:- The three planets Uranus , neptune, pluto were discovered by americas curie but father mendel got noble price and lakh dollars for it
the best ! thank you !
Californium and Einsteinium are missing
1st group:HaLiNa ke RbCs Farar.
please improve
Group 16 . Oye sallu Seth ki tange pakad.
Im in 10th I have very good suggestions here it goes ( Group Wise )
1) HeLiNa Ka Ruby Case Fraud
2) Beta Mange Car Saare Baap Raazi
3) Science Y La American
4) TiZr Half Fry Rf
5) Vo Nobita Dumb Hai
6) Chromo Waala Swag
7) Mohan Technician Rehta Sabka Bhai
8) France Roye Osama Hasse ( Just for Learning, No Offence )
)
9) Coi Rohan ko Irritate Mat Karo
10) Nidhi Prasad ka Phattu Disco
11) Copper Silver Gold Rangeen
12) Zinc Cade Mercury Cn( Se )
13)Bal Ganesh in Thailand Uut
14)Car Si( Se ) Gaye Sri Lanka( Sn ) Punjab(Pb) Finland( Fl )
15) Nana Papa Aunty Sab Bimar
16) OS Se Teri Pooja
17) Fridge Cools Bread Ice At Home
18) Helium Ne Argon Ka( Krypton ) Xedon Redon Kar Diya
Thanks I Hope u will love it
nice,cool way of learning
Napass sab bindass- N, .P, As ,Sb, Bi.
news paper aaj sab bikaga
for group 2- beta mange kanya sundar bap roye
For nobel gases…
Heena neena aur karena( ka) x ray rangeen
tips
first group
Li,Na-Lina
k-Ke
Rb,Cs-RBCs
Fr-Farar
read as: lina ke RBCs farar
1st Group, Hui Liza na kabhi Rubina se Friend.
2nd Group, Beta Mughalon Ka Sir Ba da.
3rd Group,
Vanshika nahibhai Tazeen Dumb Group 5
Cristina Moti With Sonam Gupta Group Six
Group 6
Monday Tak Raho bhai, group 7
Naina patel asif sab bimar
Naina patel asif sab bimar group 15
group 15 Nepal Pakistan Australia Sab Bike
There is a correction required in this sir… the symbol for barium is Ba not Br..
Thank you for the rest
In 2nd group barium has symbol Ba
It is not Br…..
So it is also read as
Beta Maange Car Sundar Baap Razee
made by those who are free of time.Could use this time for better
Is there any mnemonic for d and f-block elements in English?
Really great post
you just made my day!!!!
Group 13 more easy way – B Al Ga In Tl [Read as: BAl(hairs) GaIn Tel(oil) OR Baal Gain Tel ]
15th gp : Nana Patekar pass sab bindadd
2nd gp : Beta mange Canya Sundar Baap Razi
one more for 18th: Heena Neena Aur Kareena Xerox Rangeen
FULL mnemonic will be
THode PAhelwaan Unse NPtenge (TH PA U NP)
PUrane AaM Caam BKenge (PU AM CM BK)
CaFe EaSt FM Music Nhi LagaRa (CF ES FM MD NO LR )
Actinoid series-
Ac Th Pa u Np
Actinium Thorium Protactinium Uranium Neptunium
ACt THori Protactive aur Natural thi
Pu Am cm Bk Cf
Plutonium Americium Curium Berkelium Californium
Pluto American Car m Baith K California gya
Es Fm Md No Lr
Einstienium Fermium Mendelerium Nobelium Lawrencium
Einstein Farari m Mendeleev ke sath Nobel prize Laya
For 18 grp
hena neena aur kareena x ray rangeen
He
Ne
Ar
Kr
Xe
Rn
some tips
Scandium (Sc), Titanium (Ti), Vanadium (V), Chromium (Cr), Manganese
(Mn), Iron (Fe), Cobalt (Co), Nickel (Ni), Copper (Cu) and Zinc (Zn).
Mnemonics:Sachin(Sc) Tendulkar(Ti) World(Va) Cricket(Cr) Me(Mn) Fir(Fe) Koi(Co) Naya(Ni) Cup(Cu) Jetenga(Zn)
Yttrium (Y), Zirconium (Zr), Niobium (Nb), Molybdenum (Mo), Technetium
(Tc), Ruthenium (Ru), Rhodium (Rh), Pd (Palladium), Silver (Ag) and
Cadmium (Cd).
Yaari(Y) Zara(Zr) Nibhana(Nb) Maut(Mo) Tak(Tc) Rukavatein(Ru) Raahme(Rh) Pade(Pd) Agar(Ag) Koi(cd)
&
HaLiNaKi Rabdi Csti Free
Beta Maange Car Scooter Baap Roye
Group 15 Nepal pakistan AuStralia SaB BIkhari
5d series : la hafta warna osama idhar pitayi aur hogi
For first D series
Science liya hai to Tv V Cr Magazine FeCo Ni Cu nz(Zn.)
Sc Ti V Cr Mn Fe Co Ni Cu Zn.
Electronegativity order – phone kal bura aaya sun kar hadkaya Papa ne bol beta lina kon h
GROUP 13 – B AL GAngadhar INdian TiLak and +latest NeHru!
This is really an interesting and thoughtfully post for back benchers. I also with those back benchers. Because i was also a back benchers team member.
U can learn the reactivity series as follows
Kedar-k
Nath-na
Ka-ca
MaaliMg
Aloo-Al
Zarra-Zn
Feike-Fe
Se-Sn
Pakata-Pb
H-H
Kyu-Cu
Hai-Hg
Ai-Ag
Sa-Au
FOR D BLOCK ELEMENT FROM La to Hg
La Hf Ta W Re Os Ir Pt Au Hg
“(La)st Half (Ta)ste (W)as (Re)latively Odd ( Ir)ritating pathetic” — aunt mercury
hai hey lijiye b bc news o f ne ne margayi all siniar public school closed aur kya karega
–{trick to learn 20 element}
science teacher vineeta criplani mange fees copy ni cu zinc
–{21 to 30 }
In actinides Californium and Einsteinium is not present above just before fermium .
Gp-1 He,Li,Na,K,Rb,Cs,Fr
HeLiNa kabra se farar
HeLiNa (helena- name) kabra (K& Rb) se (Cs- sounds similar to se) Farar
Gp-2 Be,Mg,Ca,Sr,Ba,Ra
Beta Mange Car Sundar, Baap Raazi!
Gp-13 B,Al,Ga,In,Tl
Babu Ali Gaye India Thailand!
Gp-14 C,Si,Ge,Sn,pb
College Students Get Some Problem
Gp-15 N,P,Ar,Sb,Bi
Nana, Papa, aunty (aarti name) sab bimar
Or
News Paper Aaj Sab Bikega
Gp-16 O,Si,Se,Te,Po
Oh Sipahi! Sent tera police.
Gp-17 F,Cl,Br,I,At
Face CBI at home
Gp-18 He, Ne, Ar, Kr, Xe, Rn (noble gases)
Hai Nanapatekar Aaj Ka Zinda Raja
Or
Heena, Neena, Aur Kareena sexy & Romantic
Gp-1 He,Li,Na,K,Rb,Cs,Fr
HeLiNa (helena- name) kabra (K& Rb) se (Cs) Farar
Gp-2 Be,Mg,Ca,Sr,Ba,Ra
Beta Mange Car Sundar, Baap Raazi!
Gp-13 B,Al,Ga,In,Tl
Babu Ali Gaye India Thailand
Gp-14 C,Si,Ge,Sn,pb
College Students Get Some Problem
Gp-15 N,P,As,Sb,Bi
Nana, Papa, aunty (aarti name) sab bimar
Or
News Paper Aaj Sab Bikega
Gp-16 O,Si,Se,Te,Po
Oh Sipahi! Sent tera police.
Gp-17 F,Cl,Br,I,At
Face CBI at home
Gp-18 He, Ne, Ar, Kr, Xe, Rn (noble gases)
Hai Nanapatekar Aaj Ka Zinda Raja
Or
Heena, Neena, Aur Kareena sexy & Romantic
Another for gp.15
Nana patekar Aishwariya sabh badtameez
Yari(Y) Zara(Zr) Nibha(Nb) Maut(Mo) Tak(Tc) Rukawat(Ru) Rah(Rh) me Padegi(Of) Aage(Ag) Cadbury(Cd) Milegi
One more easier way to learn 3d series
Sc – Sachin
Ti – Tendulkar
V – very
Cr – crazy
Mn – man
Fe, Co – feco
Ni – nickel
Cu – copper
Zn – zinc
Give some more nd also very simple