Mole – Ek anmol Concept
Hello folks, I know many of you are already frustrated with the 1st chapter of Class 11th “Some basic concepts of Chemistry†which has bored you Since 1.5 months with the calculations of many concentration terms i.e. Molarity, Molality, Normality, Mole fraction, Formality, H2O2 strength etc. etc. (Chill ..I know there must be some who still haven’t come across these terms yet but you surely will)
But trust me there are students who are enjoying that chapter and the simple answer why are they loving it —–> “They are getting the correct answers of the numerical questions and you are notâ€Â: D: D. This is the truth, I know you must be knowing this too.
Have you ever thought why they get the correct answer every time but when you try , you mostly get stuck in between or maybe you don’t even know how to start. Ask yourself honestly and the answer you will get is that you are not clear with the basic stuff, funda of this chapter i.e. you don’t know about “Mole concept and its propertiesâ€Â.
It generally happens with every student who enters class 11th after giving class 10th boards (because if you remember while giving boards every topic of NCERT book was very well rattafied by you guys and you were confident ) but the moment you join class 11th , you see heap of syllabus but that’s not a problem If you just sit for 2-3 days and only practice the questions after reading this blog ,then I guarantee you ,You will be counted in the list of the Students who are loving this chapter.
The fundamental of the Chapter is “Mole Conceptâ€Â. If you are clear of this quantity, then 60% of the questions are already solved for you in this chapter.
**Before I tell you all the magic mantras and fundas related to Mole concept, I will tell you a short story.
It was the time after the board exams anda fresh beginning of new academic year in DPS R.K. Puram, New Delhi. Suraj and Nishant both were Class 10th topper with 96 % and were one of the best friends Jodi since class 6th together. Their ambitions were high and were favorites of all teachers of school.
The first day was introductory day to the new teachers and the rules, the marking scheme was told to the students. Class teacher of 11th A was Khurana Sir, English teacher.
The second day started with English Class and the second class was of Chemistry. The bell rang for the 1st class and after 5 minutes Chemistry Faculty Miss Shanaya entered the class wearing black saree. She was looking like a model in her mid-twenties. All the boys and girls in the class were totally lost looking only at her and were happy to know that on Tuesday and Thursday the Class just after boring Khurana sir will be quite happening now.
Suraj and Nishant were also amazed by the beauty as well as knowledge of Miss Shanaya and were in race to impress her.
Days passed on, and the 1st chapter was over. One day when it was Chemistry Lab period, then Suraj and Nishant had a fight on the issue that “who is favorite of Shanaya ma’amâ€Â. This matter when reached to Miss. Shanaya, She thought to resolve this problem in a fun and erudite way.
She called at once both of them and said that whosoever will answer her question will be her favorite Student else they both will leave and will never fight. They both agreed to it and both were quite happy because they both have read the topics well and were confident.
She makes them stand in front of her for 5 min and then asked the question “How many moles are in 10.00 mL of acetic anhydride? The molar mass of acetic anhydride is 102.1 g/mol?
Taking 1-2 minutes Suraj answered 4.464 * 10-4moles (dividing by 22400 mL) and was happy that he calculated first. Just after him Nishant replied 4.405 * 10-4 moles (dividing by 22700 mL) and was happy because he thought Suraj answered it in hurry and must be wrong.
After hearing both the answers Miss Shanaya smiled and said unfortunately you both are wrong, so you both may leave out and never repeat it again.
Both of them were confused, disappointed and shocked to hear that and they both moved out of her cabin.
I know you all are dying to hear the correct way to calculate the moles. Friends after reading this blog you won’t have any confusion in moles calculation and If this kind of situation happens in your school, then you will get the chance to impress Miss Shanaya in your school.
Now let me discuss this quantity for the beginners and then we will move on for experts!!
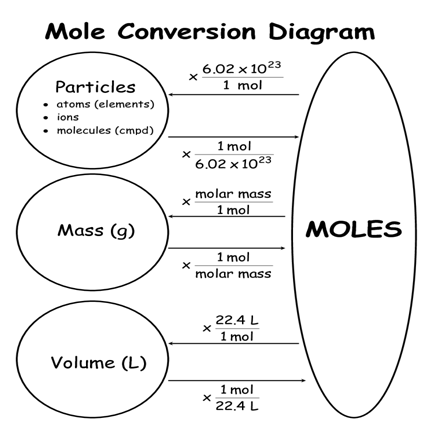
Note: I will be telling the mole calculation by 3 ways which will be interrelated.
Just imagine you are about to read a new story which will help you in studies as well. Here we go for 1st definition:
In simple words, Mole is the quantitative unit for amount of any substance and 1 mol is this number 6.022*1023(Avogadro’s constant NA). So basically if you have learnt the chapter of Set theory in mathematics, then you must see this mole as a set of collection of NAthings.
1 mole, M = {6.022*1023 things}
Here in Chemistry the things will be atoms, molecules, particles, and ions etc. etc.
So whenever someone says w.r.t. mole, then your mind should work in calculating the number corresponding to it.
For e.g. I need 1.5 moles of O atoms, automatically your mind should say I need 9.033*1023 atoms of Oxygen or I need 1.5 NA.
For those who don’t know difference between atoms and molecules, understand it with simple example:
O2 is called 1 molecule of Oxygen gas and it contains 2 atoms
So, 1 mole of O2 molecules means collection of 6.022*1023 O2and this contains 2*6.022*1023atoms.
2O means 2 atoms of Oxygen.
So, simply if you have been given the no. of atoms, molecules, particles, ions and you have been asked the no. of moles, then simply you have to divide it by Avogadro’s constant NA.
So that’s it for the 1st way definition of mole. Now we will move on to 2nd definition.
2nd definition of mole is w.r.t. Atomic mass, molar mass, formula mass, molecular mass basically related to mass.
It’s been defined with respect to Carbon as the no. of atoms present in 12 g of C-12.
So basically if we have atom then we have to take àAtomic mass
If we have molecule                        àMolecular mass
If we have compound                    àMolar mass/Formula mass.
For e.g. 1 mole of Na atoms means 23 g of Na will contain 6.022*1023 sodium atoms.
1 mole of NaCl means 58 g (23+ 35.5) of NaCl will contain 6.022*1023 NaCl molecules.
So, see how easy it is to find the no. of moles if you know the mass of the substance.
So if Someone asks you the no. of moles present if mass is given then you only have to divide it by the respective atomic mass or molar mass or molecular mass simply.
Next we will move on to the 3rd definitionand guys you have to be careful while using this definition because it sounds confusing to many students and most of the students do mistake here.Most importantly you will get the answer of the question asked by Miss Shanayain this part.
So open your eyes and ears in active mode.
3rd definitionof mole is w.r.t. the Volume of the substance.
First of all, It depends on the substance whose volume is known to you. Keep these points always in mind.
è If you have a pure liquid or solid, then you have to use its density to calculate mass and then divide the mass by molar mass.
Eg .How many moles are in 10.00 mL of acetic anhydride? The molar mass of acetic anhydride is 102.1 g/mol and its density is 1.080 g/mL?(I know you were waiting for this question)
Here, first calculate the mass = 1.080*10.00 = 10.8 g
No. of moles present = 10.8/102.1 = 0.1057
So, 0.1057moles are present.
So, Suraj and Nishant who were not knowing this concept should have asked for the density and then approached the question instead of using gaseous concepts in pure liquid. I hope you never do this mistake anytime.
è If you have a solution, then multiply the molarity by the volume in litres.
n = M * V
This you will be using while mixing the solutions and moles calculations.
ntotal= (M1V1 +M2V2)
è If you have a gas, then use Ideal gas Law
PV = nRT  =>V = nRT/P
At STP, T= 273.15K, P = 1atm, R = 0.082 Latm/mol K
So, V = 22.4 L
If pressure is 1 bar = 0.987 atm, then V = 22.7 L
So depending upon the molar volume you can also calculate the no. of moles.
Hence if you have been given volume then depending upon pressure divide either by 22.4 or 22.7 to get the no. of moles present.
With these 3 basic definitions of mole concept I hope your concept has start building up regarding the questions which involve moles.
Note: The no. of moles remains constant in a system.
All the 3 definitions are interrelated like this diagram. You can switch to any definition anytime.
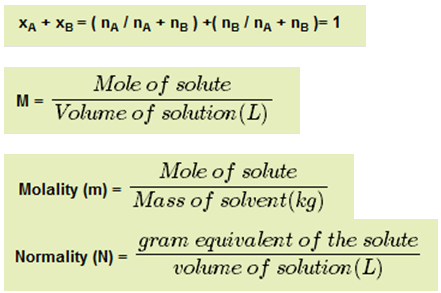
Have a look at all these formulas
In all these concentration terms you have to go via the no. of moles calculations and equivalents calculation in Normality. So you can well imagine the application of mole concept in numerical of chemistry.
And now as you have understood the basic funda of this chapter well, It will be very easy for you now to attempt the numerical questions and understand the concepts involved in concentration terms calculation.
I hope I wish you start loving mole concept just like you loved the story. 
Name: Suraj Prasad
Description: Suraj Prasad, B.Tech. Graduate in Electrical Engineering from IIT Patna and associated with askIITians Since 4 yrs. as Chemistry Expert. He believes Learning is an art which can be grasped by doing practice and get familiarize with the Science around. He is most renowned Faculty at askIITians and favorite of his students because of the awesome tips and tricks which he always provides to students in the session (not only in Chemistry but Physics and Mathematics as well). He has even made Chemistry easy and interesting for those Students who in the beginning used to hate it just by adding some humor to the subject so that student never gets bored and are able to retain the things in mind for a long time. Chemistry has become piece of cake to his students and they feel happy while solving Chemistry problems.
For the Study packages go through the below links:


wao what a sushmita !!! anmol.. truely!!!