ALKANES-SIMPLIFIED
Have you ever heard of organic compounds?? Oh, of course you would have!!
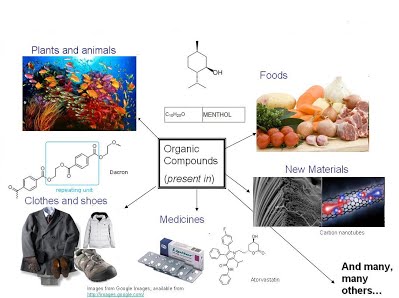
Everything which we see around is generally made up of organic compounds. The drugs we take when we feel ill, the clothes we wear (polymers), the food we take (carbohydrates, fats, vitamins etc.), dyes , soaps, detergents, plastics etc.
Organic Compounds have vast usage but yet its chemistry, undoubtedly, has always been the biggest challenge in our life. No matter how hard we try, we always tend to forget the reactions involved, the nomenclature part and isomerism in Hydrocarbons and this drives us crazy.
But the good news is that Madam Simi has brought a solution for this problem… Yuppiee !!!
Organic Chemistry is no more a challenge guys, rather it’s fun. I bet after reading this article you won’t find Hydrocarbons tougher anymore. So come on let’s begin our roller coaster hydrocarbon ride.
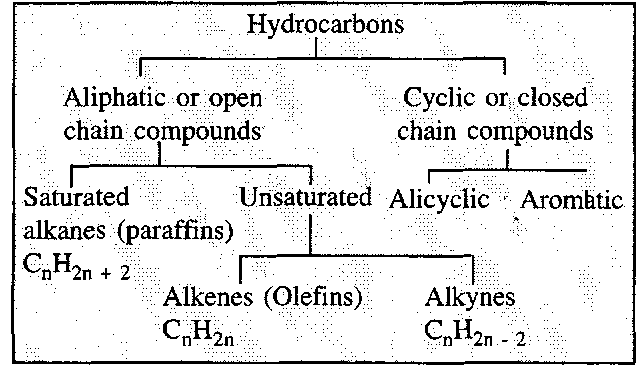
The first thing we need to understand is… What exactly are hydrocarbons and what are it’s different classification??
Now, A hydrocarbon is classified as any organic compound which is made up of nothing but Carbon and Hydrogen. There can be a possibility of double or triple bond in between two carbon atoms.
And, Here’s the classification of Hydrocarbons:
Now after doing and knowing the different kinds of hydrocarbons next major task is to understand the nomenclature… phew!! Since we are well aware of the fact that we have various types of organic compounds and it’s difficult to remember each and every name. Moreover if some compound is called ‘X’ in India it might be known as ‘Y’ in some other country and in order to call off this kind of ambiguity IUPAC (International Union of Pure and Applied Chemistry) has found a solution for it.
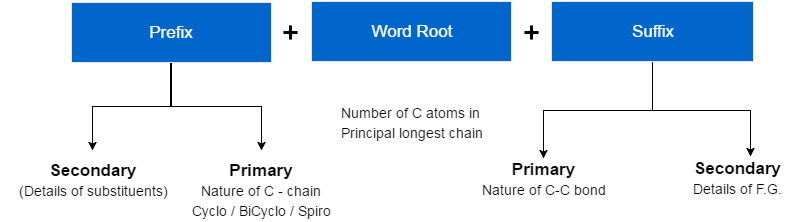
It’s very easy to understand how we give nomenclature to a compound. Here’s the basic structure :-
Now the question arises what are these terms called word root , suffix and prefix and how are they used in nomenclature. So, first let’s know about word root.
Number of Carbons in parent chain                                 Word root
                         1C                                                                          Meth
                         2C                                                                          Ethyl
                         3C                                                                          Prop
                         4C                                                                          But
                         5C                                                                          Pent
                         6C                                                                          Hex
                         7C                                                                          Hept
                         8C                                                                          Oct
                         9C                                                                          Non
                         10C                                                                         Dec
Now as far as prefix is considered, it’s of following two types:-
- Primary Prefix-It tells about the nature of carbon chain (cyclic/acyclic)
- Secondary Prefix-It tells about details of substituents.
Now after the discussion of prefix and word root, it’s time to gather some information about Suffix which again is of two different types :-
- Primary Suffix-Nature of C-C bond
- Secondary Suffix-Details of functional group
Ooops but the functional groups are secondary suffix so that means we need to know about primary suffix as well and thus here’s the list :-
| NATURE OF BOND | SUFFIX |
| C-C Single bond | ane |
| C-C Double bond | ene |
| C-C Triple bond | yne |
| 2 C-C double bonds | diene |
| 2 C-C triple bonds | Diyne |
After knowing the basics it’s time to know about the rules for nomenclating hydrocarbons:-
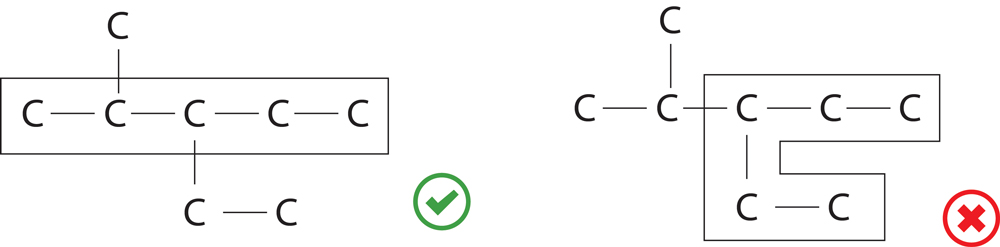
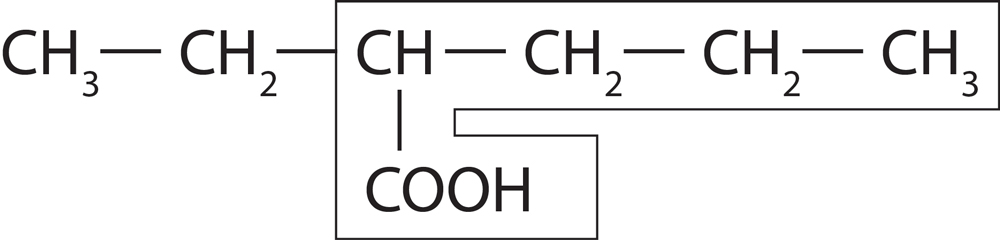
- Rule 1-Longest Chain Rule :-
Select longest possible Carbon chain as parent chain.
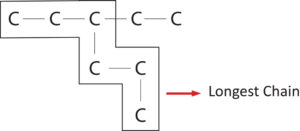 If there is more than one longest chain possible then select the chain which contains maximum number of side chain.
If there is more than one longest chain possible then select the chain which contains maximum number of side chain.
-
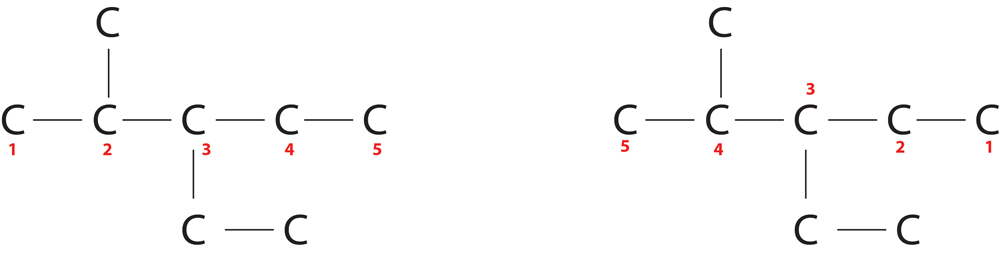
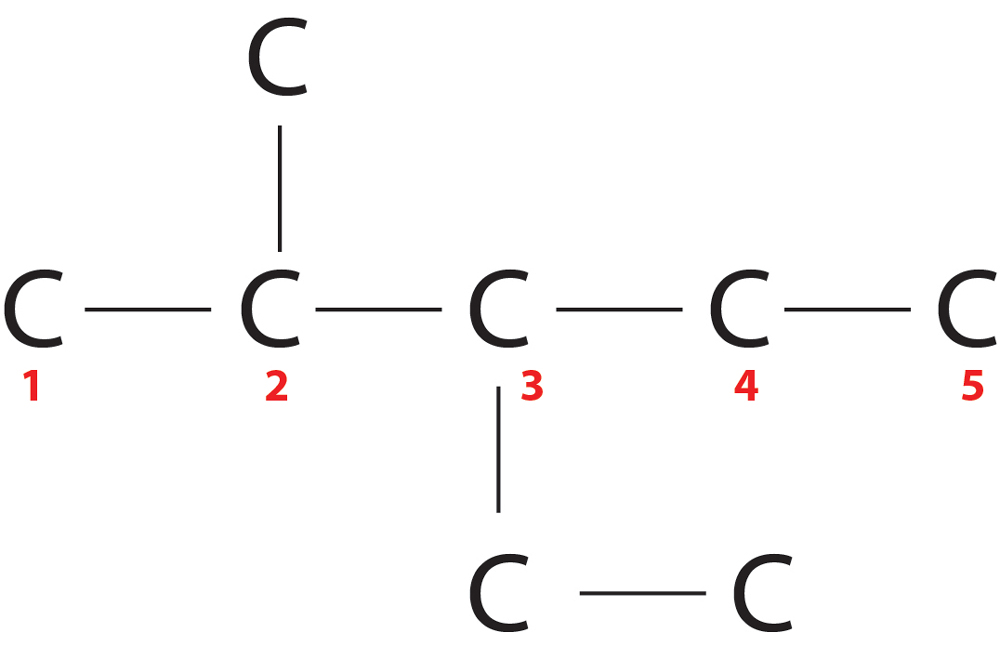
Rule 2-Lowest locant Rule :-
The substituents should get minimum sum of numbers.
In first, position of substituents=2+3=5
In second, position of substituents=3+4=7
-
Rule 3-Side chains are always written in alphabetical order
Name : 3-Ethyl-2-methyl pentane
-
Rule 4-Unsaturated carbons are preferred while numbering
If double and triple bond both are getting same numbers then, double bond is preferred over triple while numbering.![]()
-
Rule 5 – Longest chain should contain functional group if present
-
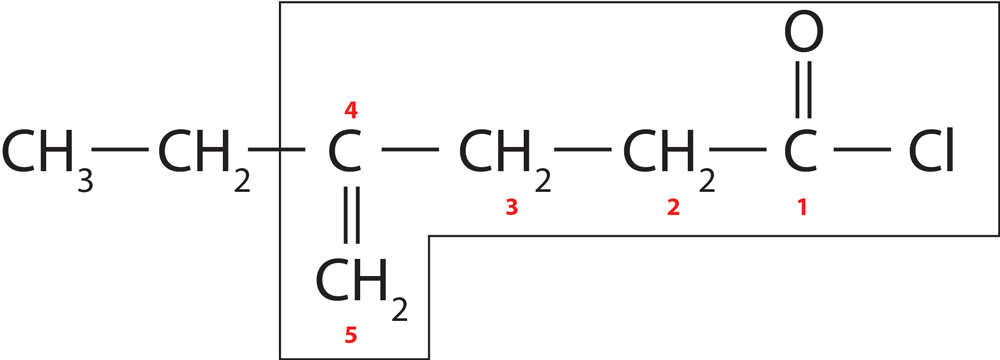
Rule 6 – Numbering starts from the side of functional group
Numbering of functional group follows the order :-
Functional group > Multiple bonds > Substituents
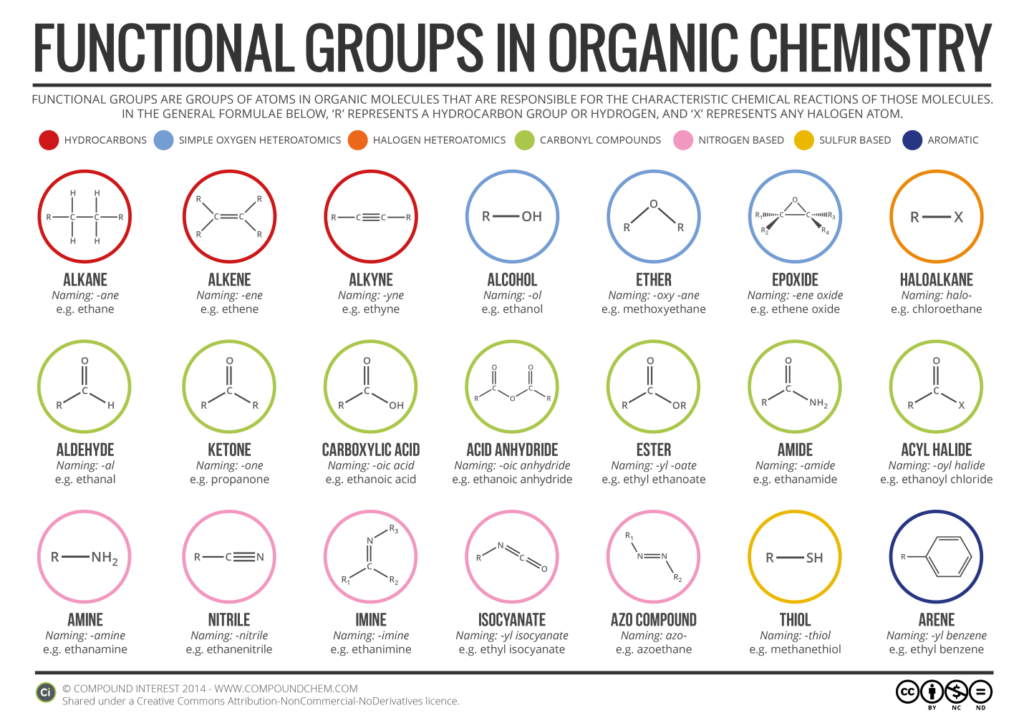
Here’s the list of all the functional groups we have :-
The priority order is as follows :-
Carboxylic acid > Sulphonic acid > Acid anhydride > Ester > Acid chloride > Amide > Cyanide > Isocyanide > Aldehyde > Ketone > Alcohol > Thio-alcohol > Amine > Alkene > Alkyne
So, that was all about nomenclature. Simple isn’t it !!
Now after knowing the names of babies of Carbon family, now let’s go to a term called ISOMERISM.
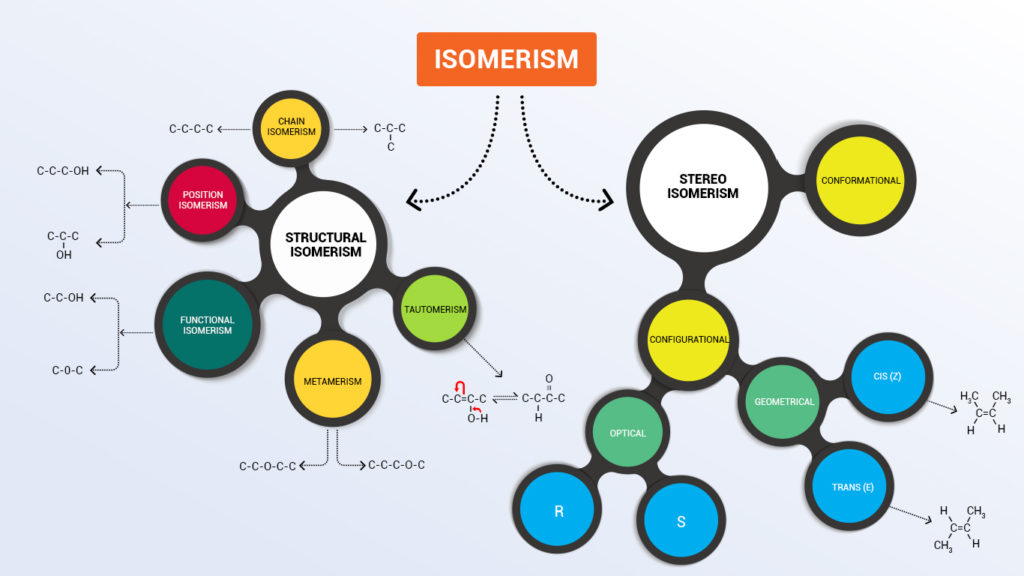
The term isomer means Compounds having same molecular formula (or we can say who have same parents) but different properties (since offspring are never same isn’t it??).The following info graphic will make you remember all types of isomerism.
Doesn’t info-graphics make things learn easy!!
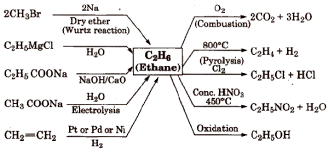
Of Corse they do…so let’s learn the chemical properties and preparations of alkane (Ethane) through another info-graphic.
Revision Notes:- General Organic Chemistry
Revision Notes:- Hydrocarbons
I hope it was nice learning. Will meet with some new concept in next blog.