Samyak Jain
Last Activity: 5 Years ago
Actually, E

is an intensive property while
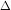
G

is an extensive property. This implies E

cannot be just added
whereas
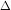
G

can be added for reactions. However, the potentials are additive when half-reations are added to yield an overall reaction but they aren’t additive when half-reactions are added to give third half-reaction.
.

= -nFE

G
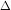 In 2014 JEE Main (offline), the two half reactions were added to giveoverall reaction (in which no electrons were present). So E
In 2014 JEE Main (offline), the two half reactions were added to giveoverall reaction (in which no electrons were present). So E could be added.But here the two reactions yield third half reaction (in which electron is present) and thus we need to proceed through
could be added.But here the two reactions yield third half reaction (in which electron is present) and thus we need to proceed through 










