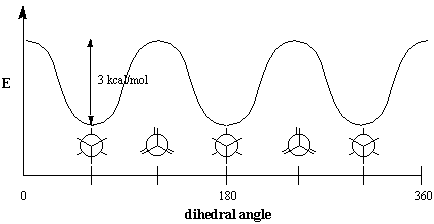
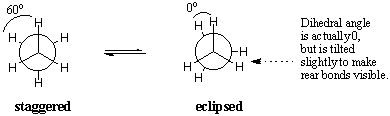Ask a Doubt
Get your questions answered by the expert for free
Enter text here...
Other Related Questions on Organic Chemistry

'x' gm of a compound A3B2C5 contains 'y' gm of A atoms Using above informationMatch the following
Organic Chemistry
0 Answer Available
Last Activity: 2 Year ago(s)

Dr. Ronald Lance is the greatest spell caster in the world also referred to as the most powerful saiki in the world by those who have used his powerful magic spells, weather you aiming at fixing your love, relationship or marriage, Ronald Lance is a real spells caster for your needs, bear in your mind that you are about to get in touch with our very experienced spells caster verified for casting authentic spells Call/Whats App+27634928462
Organic Chemistry
3 Answer Available
Last Activity: 2 Year ago(s)

What are the chapters we need to study before alcohols, phenols and ethers to understand it ?
Organic Chemistry
4 Answer Available
Last Activity: 2 Year ago(s)

Plz answer this query.....................................................................................................
Organic Chemistry
0 Answer Available
Last Activity: 2 Year ago(s)

how to represent the possible fischer projections for 2-bromohexane
Organic Chemistry
1 Answer Available
Last Activity: 2 Year ago(s)