Sweta Mishra
Last Activity: 7 Years ago
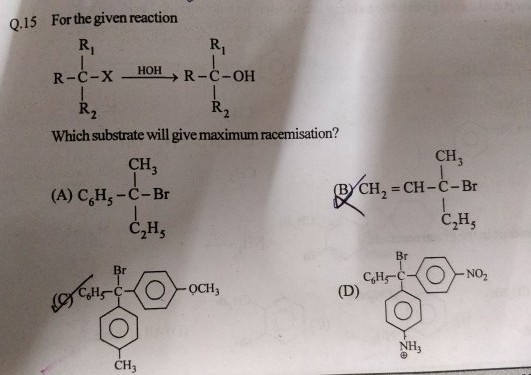
The answer is c. Racemization occurs in SN1 reactions. Intermediate of such reaction is a carbocation which is planar in shape. Now we know that greater the stability of carbocation, the greater will be the chance of SN1 reactions. Out of given choices, halide that is shown in option c will make more stable carbocation because of delocalization of positive charge in benzene ring with electron releasing group at para position. Thus it will give maximum racemization. It depends upon substrate, intermediate type of solvent.