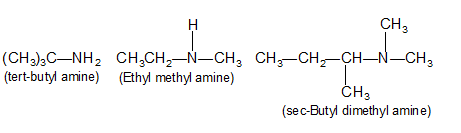Amines
Table of Content |
Amines play a vital role in medicinal chemistry. As our body is having a lot of amino acids, these amines help in the regulation of our body. It is also believed that, vitamins were named keeping vital-amines in mind (though many vitamins don't have nitrogen at all). Similarly to produce several synthetic dyes, pigments the amines are used. Therefore, the amines are very important in our everyday life. 
Structure of Amines
Amines are the alkyl (or) aryl derivatives of NH3.
![]()
The general formula of amine is R3N, where R is an alkyl (or) aryl group or hydrogen In amines one or more Hydrogen atoms of ammonia are replaced by Alkyl or aryl groups. Here nitrogen atom of amines is like that of NH3, it is sp3 hybridised. The three Alkyl groups (or hydrogen atoms) occupy corners of a tetrahedron, one of sp3 orbital occupying the unshared electron pair directed towards the other corner. We say shape of amine as “Trigonal Pyramidal”.
Amines are classified as primary, secondary (or) tertiary according to the number of groups attached to the nitrogen atom. If one alkyl group has replaced one hydrogen atom of ammonia, it is primary amine (amine group or amine functional group of primary amine is – NH2). Similarly, if two hydrogens are replaced, it is secondary amine and if all the three are replaced, it is tertiary amine (e.g trimethyl amine).
Ammonia 1O Amine 2o Amine 3o Amine
Nomenclature of Amines
Nomenclature of amines is quite simple. Aliphatic amines are named by naming the alkyl group (or) groups attached to nitrogen , and following that by the word amine for example alkyl amine (methyl amine, ethyl amine) & benzyl amine.
More complicated amines are often named as prefixing amino - (or-N-methylamino -, N-N, diethyl amino -, etc) to the name of the parent chain.
Aromatic amines - those in which nitrogen is attached to an aromatic ring - are generally named as derivatives of the simplest aromatic amine, aniline.
Salts of amines are generally named by replacing - amine by - ammonium (or - aniline by - anilinium), and adding the name of the anion.
Physical Properties of Amine
Amines are moderately polar substances; they have boiling points that are higher than those of alkanes but generally lower than alcohols of comparable molecular weight. Molecules of primary and secondary amines can form strong hydrogen bonds to each other and to water. Molecules of tertiary amines can not form hydrogen bonds to each other, but they can form hydrogen bonds to molecules of water or other hydroxylic solvents. As a result, tertiary amines generally boil at lower temperatures than primary and secondary amines of comparable molecular weight. Therefore, the order of boiling points of isomeric amines is as follows.
Primary > Secondary > Tertiary
Boiling points of amines, alcohols and alkanes of almost the same.
Related Resources
We will have a detail discussion about amines in this unit under following subtopics
Refer the below mentioned links to get an immediate solution to all queries on Organic chemistry:
JEE Organic Chemistry Syllabus
Reference books of Organic Chemistry
To read more, Buy study materials of Amines comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More