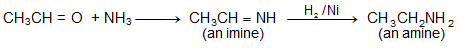Preparation of Amine
Table of the Content |
|
|
Ammonolysis of Alkyl Halides
 Many organic halogen compounds are converted into amines by treatment with aqueous (or) alcoholic solution of ammonia. This reaction is generally carried out either by allowing the reactants to stand together at room temperature (or) by heating them under pressure. Displacement of halogen by NH3 yields the amine salt, from which free amine can be liberated with hydroxide ion.
Many organic halogen compounds are converted into amines by treatment with aqueous (or) alcoholic solution of ammonia. This reaction is generally carried out either by allowing the reactants to stand together at room temperature (or) by heating them under pressure. Displacement of halogen by NH3 yields the amine salt, from which free amine can be liberated with hydroxide ion.
CH3Cl + NH3 → CH3NH3Cl  CH3NH2 + Cl– + H2O
CH3NH2 + Cl– + H2O
The above reaction is a class of substitution reaction, which we know as nucleophilic substitution.
Ammonia can act as a nucelophile and it can also act as a base. If ammonia acts a nucleophile substitution takes place, CH3CH2CH2Br + NH3 → CH3CH2CH2NH2 + HBr
And, if ammonia acts as a base, elimination takes place. (CH3)3CCl + NH3 → (CH3)2C=CH2
It is very evident that primary alkyl halides under go substitution very easily than tertiary alkyl halides, which undergo elimination very easily. Reaction with alkyl halide, yield a mixture of primary, secondary and tertiary amines and also a quaternary ammonium salt( which caannot be considered as amine)

This is because the primary amine formed again acts as a base and keep on reacting with the alkyl halide untill off the hydrogen atoms of amine gets replaced with alkyl groups. However, primary amine is obtained as a major product by taking large excess of ammonia.
Reduction of Nitro Compounds
Nitro alkanes can be reduced quantitatively to their corresponding amines
Nitro compound can be reduced in two general ways: (A) by catalytic hydrogenation using molecular hydrogen, or (B) by chemical reduction, usually by a metal and acid.
This method cannot be used when the molecule also contains some other easily hydrogenated group, such as a Carbon carbon double bond. Chemical reduction is most often carried out by adding hydrochloric acid to a mixture of the nitro compound and metal, usually granulated tin or iron.
Reduction of Nitriles
Alkyl and aryl cyanides (nitriles) can be reduced to their corresponding primary amines using lithium aluminium hydride (LiAlH4) or catalytic hydrogenation.
This reaction is used for ascent of amine series, i.e. for preparation of amines containing one carbon atom more than the starting nitrile.
Reduction of Amides
Amides can directly be converted into their corresponding amines. This reaction is carried out by treating the amide with a mixture of base and bromine (KOH + Br2). This reaction is called as Hofmann Bromamide reaction.
The reaction is as follows,
RCONH2 + Br2 + 4KOH → RNH2 + K2CO3 + 2KBr + 2H2O
Here we can see that the amine formed has one carbon less than that of the corresponding amide. Due to the loss of carbon atom, this reaction is also called as Hofmann degradation of amides.
The mechanism of the reaction is as follows:

Apart from this, amides can be dehydrated by P2O5 to their corresponding nitriles and nitriles can then be reduced.

By this method you are retaining the number of carbon atoms in both amide and the amine
Action of Ammonia on Alcohol
This method yields a mixture of 1o, 2o, 3o amines and 4o salts which are separated from each other by means of Hinsberg method, Hofmann method and fractional distillation. However, amines can be prepared in good yield by using excess of ammonia.
ZnCl3
ROH + NH3 ———> RNH2 + H2O
300oC
From Carbonyl Compounds
While studying carbonyl compounds we have seen that carbonyl compounds can be converted into any other functional group. How are we converting carbonyl group into amino group?
See, the following sequence,
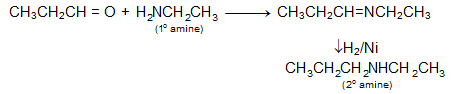
The reactions are clear and simple so that, we can get an amine from carbonyl compound just by reductive amination (amination and reduction).
Using this reductive amination we can go from 1° amine to 2° amines. How? Look at the following reaction.
Curtius Reaction
?Amines can be prepared by treating acid chloride with sodium azides the isocyanate thus formed is decomposed with treatment of water and amines are obtained.
Schmidt Reaction
?Hydrozoic acid reacts with carboxylic acid in presence of a mineral acid to give amines.
RCOOH +NH3  RNH2 + CO2
RNH2 + CO2
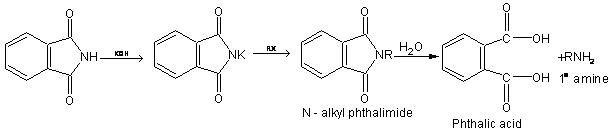
Gabriel Phthalimide Synthesis
This method is used for the preparation of primary amines. Phthalimide on treatment with ethanolic potassium hydroxide forms potassium salt of phthalimide which on heating with alkyl halide followed by alkaline hydrolysis produces the corresponding primary amine.
Aromatic primary amines cannot be prepared by this method because aryl halides do not undergo nucleophilic substitution with the anion formed by phthalimide.
Methods yielding primary amine only
1. By reduction of nitroalkanes:
C2H5NO2 + 6[H]  C2H5NH2 + 2H2O
C2H5NH2 + 2H2O
C6H5NO2 + 6[H]  C6H5NH2 + 2H2O
C6H5NH2 + 2H2O
2. Mendius reduction of alkyl cyanides:
CH3CN + 4H  CH3CH2NH2
CH3CH2NH2
Reduction of alkyl isocyanides with Na/C2H5OH gives 2o amines eg.
CH3NC  CH3CHCH3
CH3CHCH3
3. By reduction of amides and oximes:
ROCONH2  RCH2NH2
RCH2NH2
Cyclohexanone Cyclohexanoxime Cyclohexylamine
4. By Hofmann bromamide reaction:
CH3CONH2 + Br2 + 4KOH ——> CH3NH2 + 2KBr + K2CO3 + 2H2O
C6H5CONH2 + Br2 + 4KOH ——> C6H5NH2 + 2KBr + K2CO3 + 2H2O
5. By Gabriel phthalimide reaction:
Potassium phthalimide formed after reaction of phthalimide with KOH, on heating with alkyl halide gives N – alkyl phthalimide. This on hydrolysis with 20% hydrochloric acid under pressure gives 1° amine.
6. By action of chloramine on Grignard’s reagent:

7. By decarboxylation of amino acids:
H2NCH2COOH H2NCH3
H2NCH3
Glycine Methylamine
8. By Wurtz method:

CH3 – N = C = O + 2KOH ———> CH3NH2 + K2CO3
Methyl isocyanate
9. Schmidt reaction:
CH3COOH + HN3  CH3NH2 + CO2 + N2
CH3NH2 + CO2 + N2
Hydrazoic acid Methylamine
Methods giving secondary amines only
1. By reduction of alkyl isocyanide with sodium and ethanol
CH3NC + 4H  ? CH3NHCH3
? CH3NHCH3
2. By heating an alcoholic solution of 1o amine with alkyl halide
C2H5NH2 + lC2H5 ————> (C2H5)2 NH
Dimethylamine
3. By hydrolysis of p – nitroso dialkyl aniline with boiling alkali
Methods giving tertiary amine only
1. By heating alcoholic solution of NH3 with excess of alkyl halide -
3CH3l + NH3 → (CH3)3 N + 3Hl
Trimethylamine
2. By decomposition of tetra – alkyl ammonium hydroxide

(CH3)4 N+ OH–  (CH3)3 N + CH3OH
(CH3)3 N + CH3OH
Related Sources
Refer the below mentioned links to get an immediate solution to all queries on Organic chemistry:
JEE Organic Chemistry Syllabus
Reference books of Organic Chemistry
To read more, Buy study materials of Amines comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More