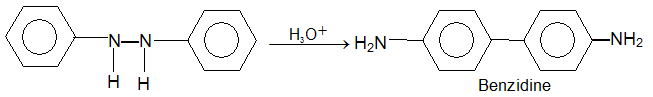Chemical Properties of Amines
Table of Content |
|
|
Basic Nature of Amines
 Like ammonia, amines are converted into their salts by aqueous mineral acids and are liberated from their salts by aqueous hydroxides. Thus , like ammonia, amines are more basic than water and less basic than hydroxide ions.
Like ammonia, amines are converted into their salts by aqueous mineral acids and are liberated from their salts by aqueous hydroxides. Thus , like ammonia, amines are more basic than water and less basic than hydroxide ions.
Amines turn red litmus blue and also combine with water and mineral acids to form corresponding salts.

When the amine salts are treated with strong bases like NaOH, the parent amines are regenerated.
RN+H3Cl– + OH– → RNH2 + H2O + Cl–
Amine salt Amine
(Soluble is water) (Insoluble in water)
Further, due to basic character amines react with auric and platinic chlorides in presence of HCl to form double salts.
RNH2 +PtCl4+2HCl --> (RNH3)2+PtCl62-
Chloroplatinic acid
These double salts decompose on ignition to pure metal, therefore, the formation and decomposition of the double salts is used for determining the molecular weight of amines.
Alkylation of Amines
?Like ammonia, an amine can react with alkyl halide to form next higher class of amine. Here, again it is the presence of electron pair on nitrogen which makes amine to behave as nuclephile and alkyl halide thus undergo nucleophilic substitution reaction.
RNH2 RNHCH3
RNHCH3 RN(CH3)2
RN(CH3)2 RN+(CH3)3I
RN+(CH3)3I
10 amine 20 amine 30 amine Quaternary ammonium iodide
Quarternary ammonium salts are useful in synthetic organic chemistry as phase-transfer catalysts and in the preparation of alkenes1
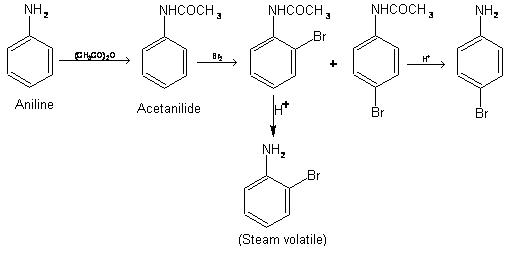
Halogenation of Amines
In order to introduce only one halogen atom, the activating effect of the –NH2 group must be lowered using acetylation.
Nitration of Amines
Direct nitration of aniline with nitric acid gives a complex mixture of mono – di and trinitro compounds and oxidation products. If however, NH2 group is protected by acetylation, main product of nitration is p – nitro derivative.
Sulphonation of Amines
Aniline reacts with conc. H2SO4 to form the salt anilinium hydrogen sulphate which on heating at 455 – 475 K gives sulphanilic acid (p – amino benzene sulphonic acid).
Sulphanilic acid exists as Zwitter ion i.e. a dipolar ion which exists in the form of internal salt structure. Such ion has positive as well as negative charge within same molecular structure.
Acylation of Amines
Reaction refers tro the reaction of amines with acyl chlorides or acid anhydrides.
Primary and secondary amines can react with acid chlorides or acid anhydrides to form substituted amides.
RNH2 + R’COCl → R’CO NHR an N-substituted amide
R2NH + R’COCl → R’CO.NR2 an N,N disubstituted amide
-
Benzoylation of Amines (Schotten Baumann Reaction)
Primary amine reacts with benzoyl chloride to give the acylated product.
R-NH2 + Cl – COC6H5 + NaOH → R-NH-COC6H5 + HCl
(Benzoyl chloride) Benzoyl alkyl amine
Carbylamine Reaction
This reaction is given only by primary amines
Primary amines when heated with chloroform and alcoholic caustic potash give isocynaides (carbylamines) having very unpleasant smell, which can be easily detected
C2H5NH2 + CHCl3 + 3KOH → C2H5NC + 3KCl + 3H2O
Ethylamine Ethyl isocyanide
C6H5 NH2 + CHCl3 + 3KOH → C6H5NC + 3KCl + 3H2O
Aniline Phenyl isocyanide
Action with Aldehyde and Ketone
Primary amines add as nucleophile to the carbonyl group of an aldehyde or a ketone to form carbinoamines, which are then dehydrated to form imines which are also known as Schiff’s bases as the final product.
C2H5NH2 + CH3CHO → C2H5NH-CH(CH3)-OH → C2H5N = CHCH3 + H2O
Ethylamine Acetaldehyde Ethylidene ethylamine
Both the addition and elimination phase of the reaction are accelerated by acid catalysis.
Secondary amines add to aldehydes and ketones to form carbinoamines which can be dehydrated to a stable product leading to a carbon-carbon double bond.
(C2H5)2NH + CH3CHO → (C2H5)2N-CH(CH3)-OH → C2H5N-CH=CH2 + H2O
Enamine
Hofmann Mustard Oil Reaction?
Primary amines when warmed with alcoholic carbon disulphide followed by heating with excess of mercuric chloride form isothiocyanates having pungent smell similar to mustard oil.
Secondary amines also react with CS2 to form dithiocarbamic acids, but the latter do not react with mercuric chloride
R2NH + S = C = S → S=C(SH)NR2 No. Reaction
No. Reaction
Tertiory amines do not react with carbon disulphide.
Reaction of Amindes with Carbonyl Chloride
This reaction is given only by primary amines.
C2H5 – NH2 + COCl2 → C2H5NCO + 2HCl
Ethylisocyanate
Hofmann Elimination
When a quaternary ammonium hydroxide is heated strongly (125° or higher) it decomposes to yield water, a tertiary amine and an alkene
This reaction is called as the Hofmann elimination. The formation of quaternary ammonium salts followed by an elimination of the kind just described and identification of the alkene and tertiary amine formed was once used in the determination of the structure of complicated amines.
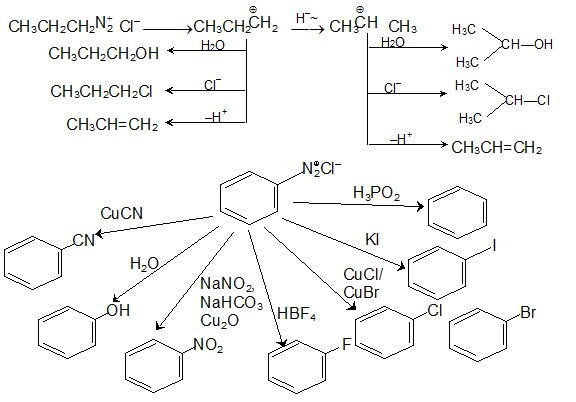
Diazonium salts of Amines
What are diazonium salts? Let us look at the name. The name suggests that, the compound has two nitrogen atoms (diazo) and the whole group has a positive charge (ium). There is also an anion to balance it (It is a salt) So, the possible structure can be: RN2+X-
How to prepare them? The preparation is quite simple if we adhere to the experimental conditions.
These diazonium salts are prepared by treating a primary amine with NaNO2 in presence of con. HCl; the temperature being 0°C. (Here the temperature has to be taken care of and if the temperature exceeds 5°C, the reaction will not take place.)

Mechanism for Diazotization
Step 1:

Step 2:
The diazonium salts of aliphatic amines are generally unstable and they decompose to give different products.
Mechanism
ArN2+ Cl– + H3PO2 reaction takes place through free radical pathway.
Chain initiation
ArN2+Cl– + H3PO2 = HCl + ArN2PH(O)OH
ArN2PH(O)OH → ArH + N2 + .PH(O)OH
Ar× + H2PO2 → ArH + PH(O)OH
ArN2+ + .PH(O)OH → ArN2· + P+H(O)OH
ArN2· → Ar. + N2 P+H(O)OH + H2O → H3PO3 + H+
Reaction of Tertiary and Secondary amines with Nitrous acid
When a tertiary aliphatic amine is mixed with nitrous acid, an equilibrium is established among the tertiary amine, its salt, and an N-Nitrosoammonium compound. Tertiary arylamines react with nitrous acid to form C-nitroso aromatic compound. Nitrosation takes place almost exclusively at the para position if it is open and if not, at the ortho position. The reaction is another example of electrophilic aromatic substitution.
N-nitrosoamines are very powerful carcinogens (cancer causing substances)
Secondary amines both aryl and alkyl react with nitrous acid to yield N-nitrosoamines. N-nitrosoamines usually separate from the reaction mixture as oily yellow liquid.
Coupling Reactions of Arene Diazonium Salts
Arenediazonium ions are weak electrophiles; they react with highly reactive aromatic compounds with phenols and tertiary arylamines to yield azo compound. This electrophilic aromatic substitution is called a diazo coupling reaction occurring mainly at p-position.
Couplings between arenediazonium cations and phenols take place most rapidly in slightly alkaline solution. If the solution is too alkaline (pH > 10), however, the arenediazonium salt itself reacts with hydroxide ion to form a relatively unreactive diazohydroxide or diazotate ion.
Hydrazo compounds are also made as follows:
Ph-NO2  Ph- NH-NH-Ph
Ph- NH-NH-Ph
Diaryl hydrazo compounds undero the benzidine rearrangement
Ring Substitution in Aromatic Amines
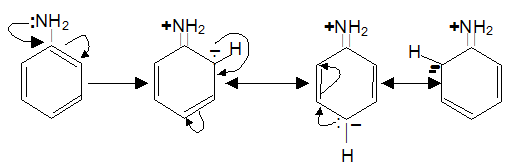
?The –NH2, – NHR and –NR2 are benzene activating groups through resonance effect of nitrogen where the lone one pair of electron of nitrogen is shifted to the benzene ring making ortho and para, position available for electrophilic attack.
The carbocation formed as intermediate are
The group – NHCOCH3 is less powerful ortho and para director because of the electron-withdrawing character of oxygen makes nitrogen a poor source of electrons. This fact is made use in preparing mono substituted aniline. The –NH2 group is such a powerful activator, that substitution occurs at all available ortho and para positions of aniline. If, however, –NH2 group is converted to –NHCOCH3, the molecule becomes less powerful activator. Hence only mono substitution products are obtained. Finally – NHCOCH3 is converted back to –NH2 by hydrolyzing with acid. This technique is especially used while nitrating aniline as strong oxidizing agent destroys the highly reactive ring.
Oxidation of Amines
?Amines are usually oxidised at N, rather than at C. Primary and secondary aliphatic amines are although oxidisable, in most cases useful products are not obtained Complicated side reaction often occurs, causing the formation of complex mixtures.

![\small (CH_3)_2CHNH_2 \xrightarrow[H_2SO_5]{Caro's Acid} (CH_3)_2C=NOH](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_smallch_3_2chnh_2_xrightarrowh_2so_5carosacidch_3_2cnoh.jpg)
![\small (CH_3)_3CNH_2 \xrightarrow[H_2SO_5]{Caro's Acid} (CH_3)_3CN=O](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_smallch_3_3cnh_2_xrightarrowh_2so_5carosacidch_3_3cno.jpg)
Aniline -X Rearrangement

Such compounds are not much stable so the group X migrates mainly at p-position.
1. Fisher-Hepp rearrangement

2. Phenylhydroxylamine - p-aminophenol rearrangement.

Nucleophilic attack by H2O at p - position.

Separation of a Mixture of Amines
a) Hinsberg’s Method
Treating a mixture of 3 amines with Hinsbergs reagent (benzene sulfonyl chloride) and finally treating the product formed with NaOH can separate the 3 class of amines.
Primary amine:RNH2 + C6H5SO2Cl → C6H5– SO2 – NH – R + HCl
C6H5– SO2 – NH – R : N-alkyl benzene sulfonamides
Dissolves in NaOH due to acidic H-attached to Nitrogen)
Secondary amine

Tertiary amine : Tertiary amines do not react with Hinsberg’s reagent.
After reacting with NaOH the aqueous layer and the second layer [Secondary and Tertiary) can be separated by ether. Aqueous layer Hydrolysed with conc. HCl gives primary amine. The ether layer is distilled and tertiary amine is distilled over. Residue hydrolysed with conc. HCl to recover secondary amine
a) Hofmann’s Method:
The mixture of amines is treated with diethyloxalate, which forms a solid oxamide with primary amine, a liquid oxime ester with secondary amine. The tertiary amine does not react.

Test for Amines
Primary amine is treated with a strong base in presence in chloroform, an isocyanide is formed and this isocyanide thus formed has a very foul smell.

Here attacking electrophile is the dichlorocarbene (:CCl2). The primary amine can be identified with its foul smell.
Secondary amine is converted into nitrosoamine by treating the amine with nitrous acid. The resultant solutions warmed with phenol and concentrated H2SO4, a brown or red colour is formed at first soon it changes to blue and then to green. The colour changes to red on dilution and further changes to greenish blue on treating with alkali.
Tertiary arylamines react with nitrous acid to form o-nitroso aromatic compound
You can also refer JEE Organic Chemistry Syllabus & Reference books of Organic Chemistry
To read more, Buy study materials of Amines comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free