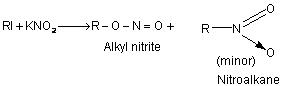Nitro and Cyno Compounds
Table of Content |
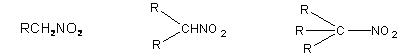
Nitro alkanes are derivatives of alkanes. They are isomeric to nitrites (esters) classified as primary, secondary and tertiary depending on the nature of carbon atom to which nitro group is linked.
Primary nitro alkane Secondary Nitro alkane Tertiary nitro alkane
—NO2 group is an ambident group. If it attacks through nitrogen. It is called nitro and if it attacks through oxygen atom, it is called nitrite. Hence nitrites and nitro compounds are isomers.
 What are ambident nucleophiles?
What are ambident nucleophiles?
Nucleophiles which can attack from two sites such as CN-, NO2- are called ambident nucleophiles
Evidences show that nitrogen is attached to one of the oxygen atoms by a double bond and to the other by a dative bond. The resonance hybrid is shown as under which confirms the spectroscopic evidence that both nitrogen – oxygen bonds have same bond length.
Resonating forms Hyrbid structure
Out of three hybrid orbitals of nitrogen one overlaps with alkyl group and two with oxygens while the unhybridized p orbital of N – atom containing a pair of electrons and lying perpendicular to the plane of hybrid orbitals overlaps sideway with half filled 2 p – orbitals of two oxygen atoms. This forms π-bond above and below the plane of molecule.
Preparation of Nitro Compounds
(i) From alkyl halides:
Alkyl halides react with silver nitrite in ethanolic solution to give nitro compounds. Alkyl nitrite is formed in minor quantity. This reaction is used to prepare 1o nitro compounds primarily while 2oand 3o halides give major proportion of alkenes due to β – elimination. Contrary to this alkali nitrites give alkyl nitrites as major product. This is due to ionic nature of alkali nitrite.
But if the reaction is carried out in solvents like DMF or DMSO, then even NaNO2 or KNO2 give good yield (about 60%) of nitro compound.
Reactions:
R—I + AgNO2 ——> RNO2 + Agl
C2H5l + AgNO2 ——> C2H5NO2 + Agl
Nitroethane
(ii) Nitration:
Nitro derivatives of aromatic compounds like nitrobenzene are produced when benzene is allowed to react with nitrating mixture.(conc. HNO3/conc.H2SO4).
Mechanism:
Generation of nitronium ion
Attack of NO2 on benzene molecule
Loss of proton:
Nitrobenzene
Direct nitration of alkane involves vapour phase nitration at high temperature.
R — H + HONO2 ———> R — NO2 + H2O
675 K low yield
Problem faced in the method is that at such high temperature, a mixture of nitro alkanes is formed due to C – C cleavage.
e.g. CH3CH2CH3 + HNO3 ——> CH3CH2CH2NO2 + CH3CH2NO2 + CH3NH2 + other products
(iii) From amines:
3o nitroalkanes can be produced as follows:
CH3 CH3
| |
CH3 ———— C ———— NH2 ————> CH3 ———— C ———— NO2 + 2H2O
| |
3o butylamine (83% yield)
Distinguish test between nitroalkanes and alkyl nitrites
1. Nitroalkane on reduction with H2/Ni produce 1o amines while alkyl nitrites produce alcohols and NH3
CH3CH2NO2 ![\small \overset{[6H]}{\rightarrow}](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_small_overset6h_rightarrow.jpg) CH3CH2NH2 + H2O
CH3CH2NH2 + H2O
1o amine
CH3CH2 — O — N = O ![\small \overset{[6H]}{\rightarrow}](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_small_overset6h_rightarrow.jpg) CH3CH2OH2 + NH3 + H2O
CH3CH2OH2 + NH3 + H2O
2.Nitroalkanes do not get hydrolysed in basic conditions while nitrites produce alcohols

CH3 O – N = O + NaOH ——> CH3OH + NaNO2
Cyanides and Isocyanides
Both alkyl cyanides (RCN) and alkyl isocyanides (RNC) are organic derivatives of hydrocyanic acid HCN. Alkali cyanides are ionic  and cyanide ion is ambident in nature (can form covalent bond either from carbon or nitrogen).AgC = N is covalent, hence lone pair on nitrogen is mainly available for covalent bond formation, resulting in predominant formation of isocyanides.
and cyanide ion is ambident in nature (can form covalent bond either from carbon or nitrogen).AgC = N is covalent, hence lone pair on nitrogen is mainly available for covalent bond formation, resulting in predominant formation of isocyanides.
Illustration . How would you account for the fact that alkyl cyanides are soluble in water but alkyl isocyanides are insoluble in water?
Solution: Alkyl cyanides possess the tendency to form H – bonding with water which is absent with isocyanides
Methods of preparation of Cyanides
1. Dehydration of Amides:

High molecular weight acid amides are dehydrated to the corresponding cyanide by heat alone.
CH3(CH3)6 OCNH2  CH3(CH2)6 CN
CH3(CH2)6 CN
2. From RX:
RX + KCN ——> RCN + KX
This method is satisfactory only if R is 1o or 2o group. If it is 3o group, then it is converted into alkene.
CH3CH2Cl + KCN → CH3CH2CN + KCl
3. By Grignard’s reagent and Cyanogen chloride reaction:
RMgCl + CICIN → RCN + MgCl2
This is best method for preparing 3o alkyl cyanides.
(CH3)3CMgCl + CICN → (CH3)3 CCN + MgCl2
4. From Diazonium salt
Methods of Preparation of Isocyanides
1. By heating an alkyl iodide with AgCN in aqueous ethanolic solution
Rl + AgCN → RNC + Agl
C2H5l + AgCN → C2H5NC + Agl
Ethylisocyanide
2. By carbylamine reaction
Heating a mixture of 1o amine and chloroform with ethanolic potassium hydroxide
RNH2 + CHCl2 + 4KOH ——> RNC + 3KCl + 3H2O
Mechanism proceeds via intermediate formation of dichloromethylene or, dichloro carbene produced from chloroform in alkaline solution. (Via a-elimination)
CHCl3 + KOH ———>KCl + H2O + : CCl2

Properties of Isocyanides
1. Alkyl isocyanides are poisonous, unpleasant smelling, with lower boiling points than isomeric cyanides.
2. RNC are not very soluble in water, nitrogen atom not having a lone pair of electrons available for hydrogen bonding.
Reactions:
1. Hydrolysis:
RNC + 2H2O  RNH2 + HCO2H
RNH2 + HCO2H
CH3NC + 2H2O  CH3NH2 + HCO2H
CH3NH2 + HCO2H
RNC are not hydrolysed by alkalis.
2. Reduction:
RNC R NHCH3
R NHCH3
2o amine
CH3NC  CH3NHCH3
CH3NHCH3
Methyl isocyanide Dimethyl amine
3. When alkyl isocyanides are heated for a long time, they arrange to form cyanide
RNC → R CN
CH3CH2NC → CH3CH2CN
4. With non metals:
(i) RNC + X2 ———> RNCX2
CH3NC + Cl2 ——> CH3NCCl2
(ii) RNC + S ———> RNCS
Alkyl isothiocyanates
CH3NC + S ———> CH3NCS
5. Oxidation with HgO:
RNC + HgO → RNCO + Hg
Akyl isocyanates
CH3NC + HgO → CH3NCO + Hg
Look here for Organic Chemistry Revision Notes, Chemistry Syllabus and Best books of Organic Chemistry.
To read more, Buy study materials of Amines comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More