Electronic Configuration
Table of Content |
Rules for filling of electrons in various orbitals
Refer to the Following Video for electronic configuration
Aufbau Principa
 This principle states that the electrons are added one by one to the various orbitals in order of their increasing energy starting with the orbital of lowest energy. The increasing order of energy of various orbital is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 5f, 6d, 7p……………………
This principle states that the electrons are added one by one to the various orbitals in order of their increasing energy starting with the orbital of lowest energy. The increasing order of energy of various orbital is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 5f, 6d, 7p……………………
How to remember such a big sequence? To make it simple we are giving you the method to write the increasing order of the orbitals. Starting from the top, the direction of the arrow gives the order of filling of orbitals.
Alternatively, the order of increasing energies of the various orbitals can be calculated on the basis of (n + l ) rule.
The energy of an orbital depends upon the sum of values of the principal quantum number (n) and the azimuthal quantum number ( l ). This is called (n +l ) rule. According to this rule,
“In neutral isolated atom, the lower the value of (n + l ) for an orbital, lower is its energy. However, if the two different types of orbitals have the same value of (n +l ), the orbitals with lower value of n has lower energy’’.
|
Type of orbitals
|
Value of n
|
Values of l |
Values of (n+l) |
Relative energy
|
|
1s
|
1
|
0
|
1 + 0 = 1
|
Lowest energy
|
|
2s
|
2
|
0
|
Higher energy than 1s orbital |
|
|
2p
|
2
|
1
|
2 + 1 = 3
|
2p orbital (n=2) have lower energy than 3s orbital (n=3) |
|
3s
|
3
|
1
|
3 + 1 = 4
|
Pauli’s Exclusion Principle
According to this principle, no two electrons in an atom can have same set of four quantum numbers. In other words, an orbital can contain a maximum number of two electrons and these two electrons must be of opposite spin. Two electrons in an orbital can be represented by
Hund’s Rule of Maximum Multiplicity
This rule deals with the filling of electrons in the equal energy (degenerate) orbitals of the same sub shell (p, d and f). According to this rule,
“Electron pairing in p, d and f orbitals cannot occur until each orbital of a given sub shell contains one electron each or is singly occupied”.
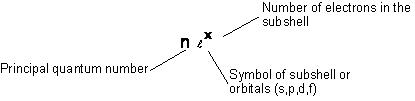
Electronic Configuration of Elements: Electronic configuration is the distribution of electrons into different shells, subshells and orbitals of an atom.
Keeping in view the above mentioned rules, electronic configuration of any orbital can be simply represented by the notation]
Alternatively:
Orbital can be represented by a box and an electron with its direction of spin by arrow. To write the electronic configuration, just we need to know (i) the atomic number (ii) the order in which orbitals are to be filled (iii) maximum number of electrons in a shell, sub–shell or orbital.
-
Each orbital can accommodate two electrons
-
The number of electrons to be accommodated in a subshell is 2 x numbers of degenerate orbitals.
| Subshell | Maximum number of electrons |
| s | 2 |
| p | 6 |
| d | 10 |
| f | 14 |
-
The maximum number of electrons in each shell (K, L, M, N…) is given by 2n2where n is the principal quantum number.
-
The maximum number of orbitals in a shell is given by n2 where n is the principal quantum number.
Exceptional Configurations
Stability of half filled and completely filled orbitals
Cu has 29 electrons. Its expected electronic configuration is
1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d9
But a shift of one electron from lower energy 4s orbital to higher energy 3d orbital will make the distribution of electron symmetrical and hence will impart more stability.
Thus the electronic configuration of Cu is
1s2, 2s2, 2p6, 3s2, 3p6, 4s1, 3d10
Fully filled and half filled orbitals are more stable
Click here to access the IIT JEE Chemistry Syllabus
To read more, Buy study materials of Structure of Atom comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free