IIT JEE Carbohydrates | JEE Carbohydrates Amino Acids Peptides
Table of Content |
|
|
The four major Biomolecules found in living organisms are Carbohydrates, Proteins, Lipids, and Nucleic Acids. Biomolecules are essential for the survival of theliving organisms.
Definition of Carbohydrates
Carbohydrates are made up of carbon, hydrogen, and oxygen. They can also be defined as Polyhydroxy Aldehydes or Ketones.
Carbohydrate Structure
Generally, word saccharides are used for carbohydrates. Saccharides includes starch, sugars, cellulose etc. Formaldehyde is considered as simplest carbohydrate. They have general formula (CH2O)n where n is three or more. They have aldehyde or ketone with hydrogen atom and hydroxyl group.
Fig.1. Structure of Carbohydrates
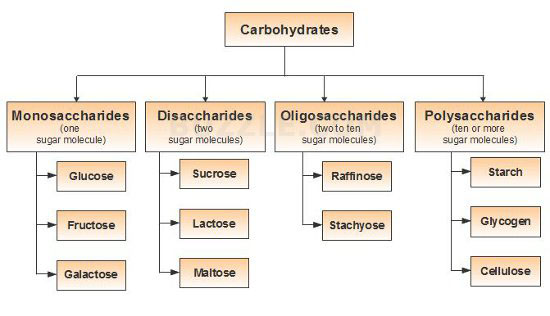
Classification of Carbohydrates / Types of Carbohydrates
Classification on the basis of number of units of sugar
Saccharides can be monosaccharides, disaccharides, oligosaccharides and polysaccharides.
The simplest saccharide or unit of saccharides are known as Monosaccharides, such as Glucose. When two monosaccharides are joined by glycosidic bond, they form Disaccharides, such as Sucrose. Sucrose is composed of Glucose and Fructose.
When 2 to 10 units of monosaccharides are joined, they form Oligosaccharides, such as Raffinose. When more than 10 monosaccharides are joined, they form Polysachharides. Such as starch, cellulose etc.
Classification of Monosaccharides on the Basis of Number of Carbons Present
If number of carbon atoms present is 6, then it is known as Hexose Sugar. For Example: Glucose.
Fig. 2. Structure of Glucose or Hexose Sugar
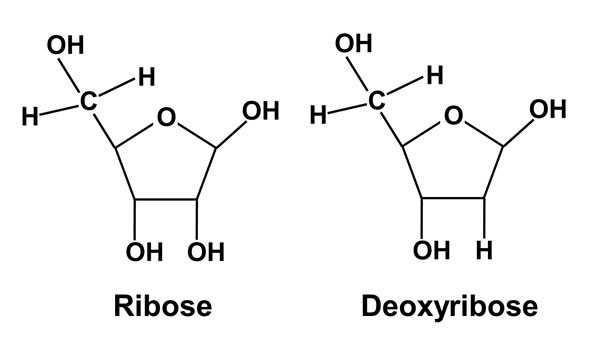
If number of carbon atoms present is 5, then it is known as Pentose Sugar. For Example: Ribose.
Fig. 3. Structure of Ribose
If number of carbon atoms present is 3, then it is known as Triose Sugar. For Example: Glyceraldehyde.
Fig. 4. Structure of Glyceraldehyde
Classification on the Basis of Functional Group Present
Aldoses contain the aldehyde group. For Example: Glucose, Galactose, Ribose, Glyceraldehyde, etc.
Ketoses contain the ketone group. For Example: Fructose
Reducing sugars contain a hemiacetal or hemiketal group. For Example: Glucose, Galactose, Fructose, Maltose, Lactose
Non-reducing contain no hemiacetal groups. For Example: Sucrose and all polysaccharides are non-reducing sugars.
Monosaccharides
-
Glucose is the most important carbohydrate needed by living organisms. It easily crosses the cell membrane. Glucose on oxidation releases energy via process known as Respiration. Its chemical formula is C6H12O6. The sugar that circulates in blood is glucose. The level of glucose determines whether the person is suffering from diabetes or not.
-
Galactose is made up of glucose and galactose. Lactose is a milk sugar. It is also a component of ABO blood groups in humans.
-
Fructose is a ketose sugar. It is 5 carbon sugar. Fructose and glucose together forms a sucrose.
-
Ribose and deoxyribose sugars are found in nucleic acids. RNA contains ribose sugar whereas DNA contains deoxyribose sugar. In deoxyribose sugar, -OH is replaced by -H atom.
Fig. 5. Cyclic Structure of Ribose and Deoxyribose
Disaccharides
-
Sucrose is the disaccharide found in plants. The end product of photosynthesis, is sucrose. It is a non-reducing sugar. The chemical formula of sucrose is C12H22O11.
-
Lactose also known as milk sugar. It is commonly found in dairy products. It is a reducing sugar. It is an isomer of sucrose.
-
Maltose is formed by joining of two glucose units via glycosidic bond. When amylase breaks down into starch, it forms maltose or malt sugar. It is also a reducing sugar.
Polysaccharides
-
Plants store food in the form of starch. Starch is made up of amylose and amylopectin. Both amylose and amylopectin are made up of glucose. Amylose is an unbranched polymer of glucose whereas amylopectin is branched polymer of glucose.
Fig. 6. Structure of Amylose and Amylopectin
-
In animals, glucose is stored in the form of glycogen. It is also made up of amylose and amylopectin. But in glycogen, branching occurs at very short distances.
Fig. 7. Structure of Glycogen
-
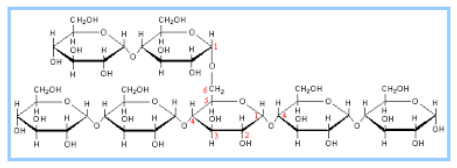
Cellulose is another polymer of glycose. It is a straight chain polymer of glucose. Plant cell wall is made up of cellulose. It provides strength and rigidity to the cell wall. Cellulose forms a tough structure due to strong hydrogen bonding between the chains of the polymer.
Fig. 8. Structure of Cellulose
Human beings are unable to digest cellulose as they do not contain any enzyme required for cellulose digestion. Ruminants have special stomach for digestion of cellulose.
Functions of Carbohydrates
-
Cellulose is present in cell wall of plants that provides and rigidity to it.
-
Lactose sugar is found in milk.
-
Chitin is also another sugar found in exoskeleton of insects.
-
It is a dietary fiber.
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More