Classification of Carbohydrates
Table of Content |
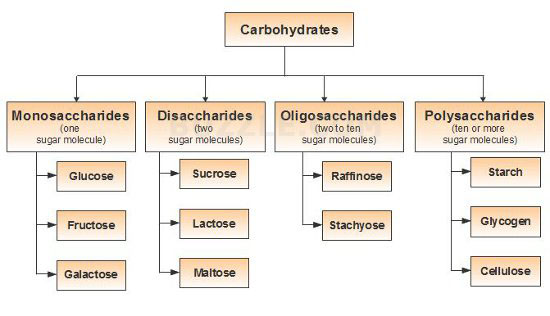
The carbohydrates are divided into three major classes depending upon whether or not they undergo hydrolysis, and if they do, on the number of products formed.
Monosaccharides
The monosaccharides are polyhydroxy aldehydes or polyhydroxy ketones which cannot be decomposed by hydrolysis to give simpler carbohydrates. Examples are glucose and fructose, both of which have molecular formula, C6H12O6.
The monosaccharides are the basis of carbohydrate chemistry since all carbohydrates are either monosaccharides or are converted into monosaccharides on hydrolysis. The monosaccharides are polyhydroxy aldehydes or polyhydroxy ketones. There are, therefore, two main classes of monosaccharides.
1.The Aldoses, which contain an aldehyde group
2.The Ketoses, which contain a ketone group 
The aldoses and ketoses are further divided into sub-groups on the basis of the number of carbon atoms in their molecules, as trioses, tetroses, pentoses, hexoses, etc. To classify a monosaccharide completely, it is necessary to specify both, the type of the carbonyl group and the number of carbon atoms present in the molecule. Thus monosaccharides are generally referred to as aldotrioses, aldotetroses, aldopentoses, aldohexoses, ketohexoses, etc.
The aldoses and ketoses may be represented by the following general formulas.

Glucose and fructose are specific examples of an aldose and a ketose.
|
Carbon Atoms |
General Terms |
Aldehydes |
Ketones |
|
3 |
Triose |
Aldotriose |
Ketotriose |
|
4 |
Tetrose |
Aldotetrose |
Ketotetrose |
|
5 |
Pentose |
Ketopentose |
|
|
6 |
Hexose |
Aldohexose |
Ketohexose |
|
7 |
Heptose |
Aldoheptose |
Ketoheptose |
Oligosaccharides
The oligosaccharides (Greek, oligo, few) are carbohydrates which yield a definite number (2-9) of monosaccharide molecules on hydrolysis. They include,
Disaccharides, which yield two monosaccharide molecules on hydrolysis. Examples are sucrose and maltose, both of which have molecular formula, C12H22O11.

Trisaccharides, which yield three monosaccharide molecules on hydrolysis. Example is raffinose, which has molecular formula, C18H32O16.
![]()
Tetrasaccharides, etc.
Disaccharides
Carbohydrates which upon hydrolysis give two molecules of the same or different monosaccharides are called disaccharides. Their general formula is C12H22O11. The three most important disaccharides are sucrose, maltose, and lactose. Each one of these on hydrolysis with either an acid or an enzyme gives two molecules of the same or different monosaccharides as shown below:

Disaccharides may also be considered to be formed by a condensation reaction between two molecules of the same or different monosaccharides with the elimination of a molecule of water. This reaction involves the formation of an acetal from a hemiacetal and an alcohol – in which one of the monosaccharides acts as the hemiacetal while the other acts as the alcohol.
Sucrose
It is formed by condensation of one molecule of glucose and one molecule of fructose. Unlike maltose and lactose, it is non-reducing sugar since both glucose (C1 - α) and fructose (C2 -β) are connected to each other through their reducing centres. Its structure is shown below:
Hydrolysis: (Invert Sugar or Invertose). Hydrolysis of sucrose with hot dilute acid yields
D-glucose and D-fructose.

Sucrose is dextrorotatory, its specific rotation being +66.5%, D-glucose is also dextrorotatory, [α]D = +53°, but D-fructose has a large negative rotation, [α]D = -92°. Since D-fructose has a greater specific rotation than D-glucose, the resulting mixture is laevorotatory. Because of this the hydrolysis of sucrose is known as the inversion of sucrose, and the equimolecular mixture of glucose and fructose is known is invert sugar or invertose.
Polysaccharides
The polysaccharides are carbohydrates of high molecular weight which yield many monosaccharide molecules on hydrolysis. Examples are starch and cellulose, both of which have molecular formula, (C6H10O5)n.

In general, the monosaccharides and oligosaccharides are crystalline solids, soluble in water and sweet to taste. They are collectively known as sugars. The polysaccharides, on the other hand, are amorphous, insoluble in water and tasteless. They are called non-sugars. The carbohydrates may also be classified as either reducing or non-reducing sugars. All those carbohydrates which have the ability to reduce Fehling’s solution and Tollen’s reagent are referred to as reducing sugars, while others are non-reducing sugars. All monosaccharides and the disaccharides other than sucrose are reducing sugars.Polysaccharides are formed when a large number (hundreds to even thousands) of monosaccharide molecules join together with the elimination of water molecule. Thus, polysaccharides may be regarded as condensation polymers in which the monosaccharides are joined together by glycosidic linkages.
Some important polysaccharides are:
1. Cellulose
2. Starch
3. Glycogen
4. Gums and
5. Pectins
6. Starch
It is a polymer of glucose. Its molecular formula is (C6H10O5)n where the value of n (200 – 1000) varies from source to source. It is the chief food reserve material or storage polysaccharide of plants and is found mainly in seeds, roots, tubers, etc. Wheat, rice, potatoes, corn, bananas etc., are rich sources of starch.
Starch is not a single compound but is a mixture of two components – amylose (10 to 20%) and amylopectin (20 to 80%). Both amylose and amylopectin are polymers of
α-D-glucose.
Amylose is a linear polymer of α-D-glucose. It contains about 200 glucose units which are linked to one another through α-linkage involving C1 of one glucose unit with C4 of the other as shown below:
Amylopectin, on the other hand, is a highly branched polymer. It consists of a large number (several branches) of short chains each containing 20-25 glucose units which are joined together through α-linkages involving C1 of one glucose unit with C4of the other. The C1 of terminal glucose unit in each chain is further linked to C6 of the other glucose unit in the next chain through C1 – C6 α-linkage. This gives amylopectin a highly branched structure as shown below.-
Hydrolysis: Hydrolysis of starch with hot dilute acids or by enzymes gives dextrins of varying complexity, maltose and finally D-glucose. Starch does not reduce Tollen’s reagent and Fehling’s solution.
Uses: It is used as a food. It is encountered daily in the form of potatoes, bread, cakes, rice etc. It is used in coating and sizing paper to improve the writing qualities. Starch is used to treat textile fibres before they are woven into cloth so that they can be woven without breaking. It is used in manufacture of dextrins, glucose and ethyl alcohol. Starch is also used in manufacture of starch nitrate, which is used as an explosive.
Refer the below mentioned links to get an immediate solution to all queries on Organic chemistry:
JEE Organic Chemistry Syllabus
Reference books of Organic Chemistry
To read more, Buy study materials of Biomolecules comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More