Peptide and Proteins
Table of Content |
|
|
Peptide and Proteins
Proteins are formed by joining the carboxyl group of one amino to the α - amino group of another acid. The bond formed between two amino acids by the elimination of water molecules is called peptide linkage.
The product formed by linking amino acid molecules through peptide linkage -CO - NH - is called a peptite. When two amino acids combined in this way the resulting product is called a dipeptide.
Peptide are further designated as tri, tetra or penta peptides accordingly as they contain three, four or five amino acid molecules, same or different. In a peptide the amino acid that contains the free amino group is called the N – terminal residue (written on L.H.S). The amino acid that contains the free carboxyl group is called the C – terminal residue (written on R.H.S).
If a large number of α - amino acids (100 to 1000) are joined by peptide bonds the resulting polyamide is called polypeptide.
While a peptide having a molecular mass more than 10,000 is called a protein.
Structure of Proteins
Primary Structure of Protien
-
This type of structure was given by Friedrich Sanger in 1953 in Insulin.
-
Primary structure is conformed by single polypeptide chain in a linear manner.
-
All amino acid are attached in a straight chain by peptide bond.
Secondary Structure of Protein
-
The fixed configuration of polypeptide skeleton is referred to as the secondary structure of protein.
-
It gives information about the manner in which the protein chain is folded and bent and also about nature of the bond which stabilizes this structure.
-
This structure of protein is mainly of two types
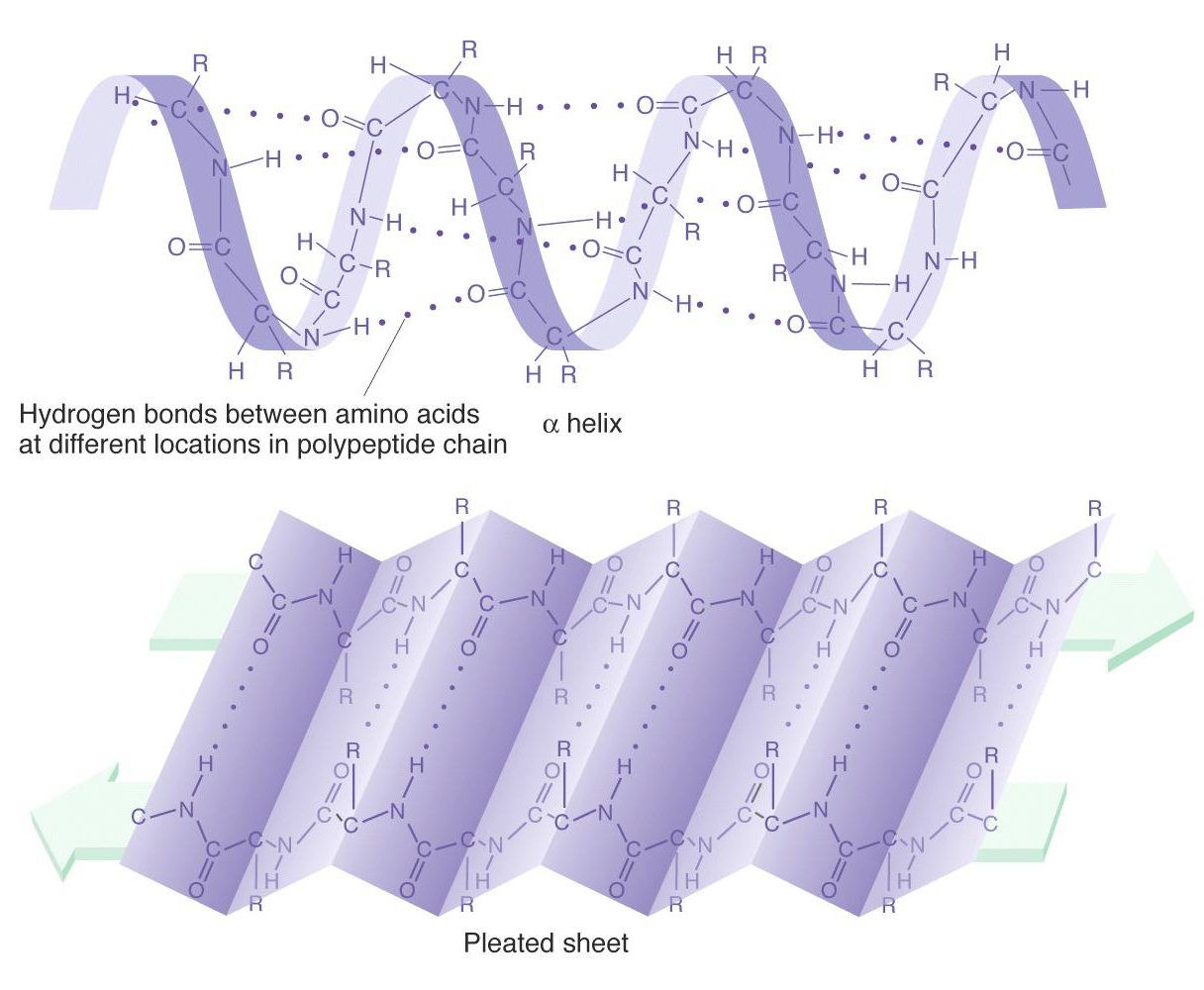
(A) α-Helix
-
The chain of α-amino acids coiled as a right handed screw (called α-helix) because of the formation of hydrogen bond.
-
The spiral is held together by H-bonds between N–H and C = O group vertically adjacent to one another.
-
X-Ray studies have shown that there are approximately 3.6 amino acid unit for each turn in helix.
-
Such proteins are elastic i.e., they can be stretched.
-
On stretching weak H-bonds break up and the peptide act like a spring.
-
The hydrogen bonds are reformed on releasing the tension.
-
e.g. Myosin, Keratin, Tropomysin.
(B) Beta-pleated sheet
-
Polypeptide chains are arranged side by side.
-
The chains are held together by a very large number of hydrogen bond between C = O and NH of different chains.
-
These sheets can slide over each other to form a three dimensional structure called a beta pleated sheet. e.g. Silk has a beta pleated structure.
Tertiary Structure of Protein
-
It refers to the arrangement and interrelationship of the twisted chain of protein into specific layer or fibres.
-
This tertiary structure is maintained by weak interatomic force such as, H-bonds hydrophobic bond, van der Waals’ force and disulphide bonds (eg Insulin). e.g. Protein of tobacco mosaic virus (TMV); Myoglobin; Hemoglobin.
Quarternary Structure of Protein
-
When two or more polypeptide chain united by the force other than covalent bond i.e., peptide and disulphide bonds.
-
It refers to final three dimensional shape that results from twisting bonding and folding of the protein helix.
-
It is most stable structure.
Refer the foolowing video for primary, secondary, tertiory and quartenary structures of protein
Classification of Proteins
There are two methods for classifying proteins.
-
Classification according to Composition
-
Classification according to Functions
Classification according to Composition
Simple Proteins
Simple proteins are those which yield only α-amino acids upon hydrolysis.Simple proteins are composed of chain of amino acid unit only joined by peptide linkage.
Examples are: Egg (albumin); Serum (globulins); Wheat (Glutelin); Rice (Coryzenin)
Conjugated Proteins
Conjugated proteins are those which yield α - amino acids plus a non protein material on hydrolysis. The non protein material is called the prosthetic group.
Example: Casein in milk (prosthetic group is phosphoric acid); Hemoglobin (prosthetic group is Nucleic acid); Chlolesterol (prosthetic group – lipid).
According to molecular shape, proteins are further classified into two types.
(A) Fibrous protein
(a) These are made up of polypeptide chain that are parallel to the axis & are held together by strong hydrogen and disulphide bonds.
(b) They can be stretched & contracted like thread.
(c) They are usually insoluble in water.
Example: Keratin (hair, wool, silk & nails); Myosin (muscle)
(B) Globular Proteins
(a) These have more or less spherical shape (compact structure).
(b) α - amino helix are tightly held bonding; H – bonds, disulphide bridges, ionic or salt bridges:
Examples: Albumin (egg)
Classification according to Functions
The functional classification includes following groups.
Structural Proteins
These are the fibrous proteins such as collogen (skin, cartilage & bones) which hold living system together.
Blood Proteins
(i) The major proteins constituent of the blood are albumin hemoglobin & fibrinogen.
(ii) Their presence contribute to maintenance of osmotic pressure, oxygen transport system & blood coagulation respectively.
Chemical Tests for Protein
Biuret test
On adding a dilute of copper sulphite to alkaline solution of protein, a violet colour is developed. This test is due to the presence of peptide (-CO-NH-)linkage.
Refer the following video for Biuret Test
Millon’s test
Millon’s reagent consists of mercury dissolved in nitric acid (forming a mixture of mercuric & mercurous nitrates). When millon’s reagent is added to a protein, a white ppt is formed, which turn brick red on heating. This test is given by protein which yield tyrosine on hydrolysis (due to the presence of phenolic group).
Nihydrin test
This test is given by all proteins. When protein is boiled with a dilute solution of ninhydrin, a violet colour is produced.
Uses of Proteins
-
Protein constitute as essential part of our food, meat, eggs, fish, cheese provide protein to human beings.
-
Casein (a milk protein) is used in the manufacture of artificial wool & silk.
-
Amino acid needed for medicinal use & feeding experiment, are prepared by hydrolysis of proteins.
-
Gelatin is used in desserts, salad’s, candies bakery goods etc.
-
Leather is obtained by tanning the protein of animal hides.
-
Hemoglobin present in blood is responsible for carrying oxygen and CO2.
-
Hormones control various process.
-
Enzymes are the proteins produces by living system & catalyse specific biological reaction.
Example:
-
Ureases (Urea → CO2 + NH2)
-
Trypsin (Protein → Amino acid)
-
Carbonic anhydride (H2CO3 → H2O + CO2)
-
Nuclease (RNA, DNA →Nucleotides)
Related Resources
To read more, Buy study materials of Biomolecules comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More