IIT JEE Proteins | JEE Alpha Helix Structure
Table of Content |
Define Proteins
Proteins are large macromolecules consisting chain of amino acids that plays an important role in many body functions. There are 22 different amino acids found.
Fig. 1. Twenty-two different Amino Acids
Structure of Amino Acids
Each Amino Acid contains carbon atom to which amino group, carboxyl group, hydrogen atom and one R group is attached. R group can be hydrogen atom or any other alkyl group. Simplest amino acid is glycine. In glycine, R group is replaced by hydrogen atom. Amino acids are surrounded by four different groups, so alpha carbon is said to be a chiral carbon. Glycine contains, two same atoms, that is, hydrogen atom. So, it is said to be achiral.
Fig. 2. Structure of Amino Acid
Peptide Bond
Each Amino Acid is joined to another amino acid by a peptide bond. Peptide bond is formed by the removal of water molecule formed by -OH from carboxyl group and -H from amino group.
Fig. 3. Peptide Bond Formation
Structure of the Proteins
The protein exists most commonly in four different forms:
Primary Structure of the Protein
The sequence of amino acids is called the primary structure of a protein. The left end is represented by the first amino acid, while the right end is represented by the last amino acid. The first amino acid is also called N-terminal amino acid. The last amino acid is called C-terminal amino acid.
Secondary Structure of the Protein
The protein is not a linear chain of amino acids rather the chain would bend at some places and even form helices. Regularly repeating local structures gives secondary structure to protein. It consists of alpha helix, beta sheet and turns.
Alpha Helix is a coiled structure in which N-H group of an amino acid forms hydrogen bond with C=O of another amino acid. The most common amino acids that forms alpha helix are alanine, methionine, asparagine etc. Proline is unable to form alpha helix because of its structure.
Fig. 4. Structure of Alpha Helix
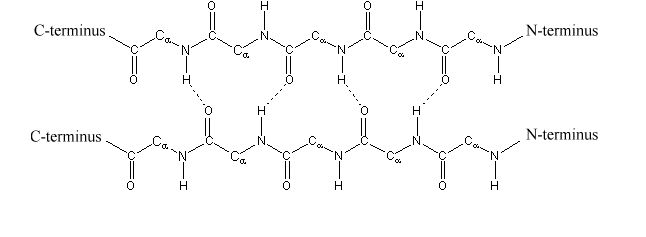
Beta sheets or beta pleated sheets consists of two beta strands joined by hydrogen bonding between the N-H group of an amino acid forms hydrogen bond with C = O of another amino acid.
Fig. 5. Structure of Beta Sheets
Tertiary Structure of the Protein
The overall shape of a protein molecule; and the spatial relationship of the secondary structures to one another; is called tertiary structure of protein. In other words, the various folds which give three dimensional appearances to protein form its tertiary structure.
Quaternary Structure of the Protein
The manner in which the individual folded polypeptides are arranged with respect to each other is called quaternary structure of protein
Protein Denaturation
Proteins has the property to get unfolded. When a protein lost its native structure, it is said to be denatured. Denatured protein does not perform its native function. Common denaturing agents are high temperature, extremes of pH, urea, detergents, etc. When denaturing agent is removed, the protein will regain its structure it is known as renaturation.
Boiled eggs become hard because egg proteins are denatured. A classic example of denaturing in proteins comes from egg whites, which are typically largely egg albumins in water. Fresh from the eggs, egg whites are transparent and liquid. Cooking the thermally unstable whites turns them opaque, forming an interconnected solid mass.
Types of Proteins / Classification of Proteins
There are two types of proteins- globular proteins and fibrous proteins.
Globular proteins are soluble proteins, that is, they are soluble in water. They have weak intermolecular hydrogen bonding. They mostly exist in three-dimensional structure.
Fibrous proteins are insoluble proteins with strong intermolecular hydrogen bonding. They generally form sheet or helixes.
Some Important Proteins
Hemoglobin is a globular protein made up of 2 alpha and 2 beta polypeptides. The central atom found is Fe2+ and prosthetic haem group. The main function of hemoglobin is to transport oxygen and carbon-dioxide in the body. One hemoglobin molecule can bound 4 oxygen atoms.
Myoglobin is another globular protein which is found in muscles. Its transports oxygen in muscles.
Collagen is a fibrous protein, made up of three polypeptides. Three polypeptides wrap around each other to form triple helix. It is an important structure protein. It is found in cartilage and connective tissue. It is also a component of tendons which connects skeleton muscles to bones.
Watch this Video for more reference
More Readings