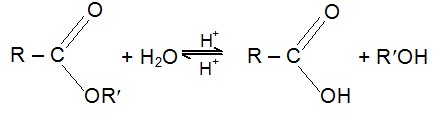Carboxylic Acid Derivatives
Table of Content |
Carboxylic acid derivatives are formed by substitution of -OH group of carboxylic acids by -X, -OR or -NH2 .So, there are four carboxylic acid derivatives. These are generally represented as R – C – Z, where Z is halogen (usually Cl), OCOR', OR' or NH2 (or NHR' or NR2').
-
When Z is halogen (usually Cl), the derivatives are called as acid chlorides i.e. RCOCl
-
When Z is —OR', the derivatives are called as esters i.e. RCOOR’
-
When Z is – O – C – R', the derivatives are called carboxylic anhydrides i.e. (RCO)2O
-
Where Z is — NH2, the derivatives are called amides. i.e. RCONH2
-
When Z is –NHR' or –NR2'they are called N – substituted amides i.e. RCONHR
Esters
What are esters?
?Esters are the derivatives of carboxylic acids which are formed by substitution of -OH group of carboxylic acid by -OR. Carboxylic acids are esterified with alcohols or phenols in the presence of a mineral acid such as concentrated H2SO4 or HCl gas as a catalyst.
What is esterification?
The process of conversion of carboxylic acids into ester by its reaction with alcohols in the presence of mineral acids is known as esterification or esterification reaction.

Esterification reaction is reversibl and the same catalyst, hydrogen ion, that catalyzes the forward reaction i.e. esterification necessarily catalyzes the reverse reaction i.e. hydrolysis.
Reactivity of alcohols for esterification reaction follows the order CH3OH > 1° > 2° > 3°.
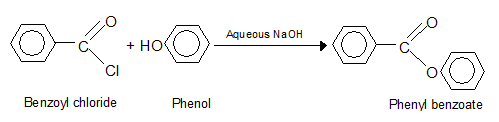
The equilibrium is particularly unfavorable when phenols (ArOH) are used instead of alcohol; yet if water is removed during the reaction, phenolic esters (RCOOAr] are obtained in high yield.
The presence of bulky group near the site of reaction, whether in alcohol or in the acid, slows down esterification (as well as its reverse, hydrolysis). and that is why the order of reactivity of carboxylic acids for esterification reaction is HCOOH > CH3COOH > RCH2COOH > R2CHCOOH > R3CCOOH
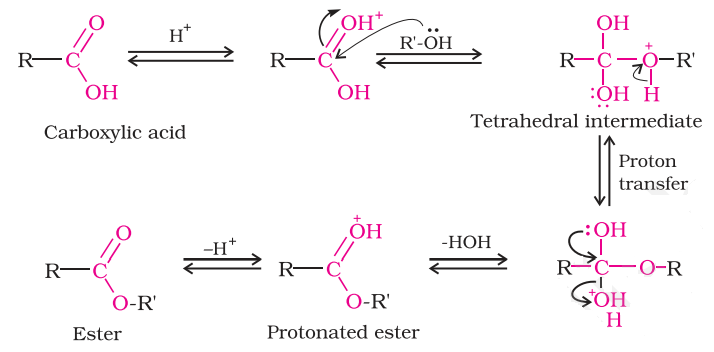
What is the mechanism of esterification reaction?
?The step in the mechanism for the formation of an ester from an acid and an alcohol are the reverse of the steps for the acid-catalyzed hydrolysis of an ester, the reaction can go in either direction depending on the conditions used. A carboxylic acid does not react with an alcohol unless a strong acid is used as a catalyst, protonation makes the carbonyl group more electrophilic and enables it to react with the alcohol, which is a weak nucleophile.
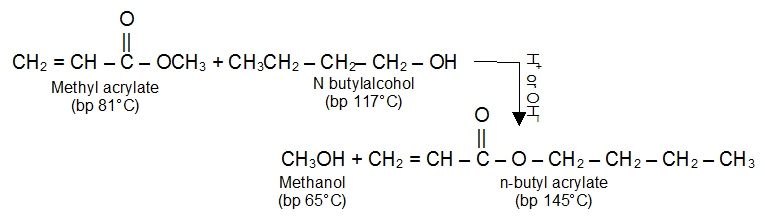
What is Transesterification?
An alcohol is capable of displacing another alcohol from an ester. This alcoholysis (cleavage by an alcohol) of an ester is called transesterification. In other words one can say that it is the process of exchanging the organic group R of an ester with the organic group R’ of an alcohol.
R-OH + R-COOR → R-OH + RCOOR
Alcohol Ester
Transesterification is often catalysed by acid (H2SO4 or dry HCl) or base (usually alkoxide ion). Transesterification is an equilibrium reaction. To shift the equilibrium to the right, it is necessary to use a large excess of the alcohol.
The difference in the boiling points of the alcohols allows the equilibrium to be shifted toward the higher molecular weight ester by distilling the methanol out of the reaction mixture.
What is Saponification?
Esters are hydrolysed by acids or alkalis. Acidic hydrolysis is reversible and hence the mechanism for hydrolysis is also taken in the opposite direction of the mechanism for esterification.
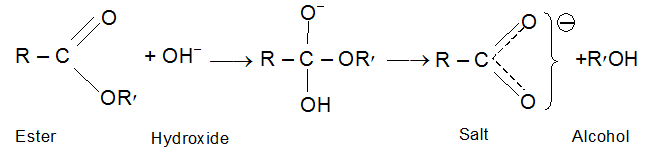
When hydrolysis is carried out with alkali the carboxylic acid is obtained as its salt. This reaction is essentially irreversible, since a resonance stabilized carboxylate anion show little tendency to react with an alcohol. Since the alkali salts of the higher acids are soaps, alkaline hydrolysis is known as saponification; Saponification is far more rapid than acid hydrolysis. If an ester is hydrolyzed in a known amount of base (taken in excess), the amount of base used up can be measured and used to give the saponification equivalent; the equivalent weight of the ester, which is similar to the neutralization equivalent of an acid.
Mechanism of Saponification
Esters undergo base promoted hydrolysis also. This reaction is known assaponification, because it is the way most of the soaps are manufactured. Refluxing an ester with aqueous NaOH produces an alcohol and the sodium salt of the acid.This reaction is essentially irreversible because carboxylate ion is inert towards nucleophilic substitution.
If an ester is hydrolysed in a known amount of base (taken in excess), the amount of base used up can be measured and used to calculate the saponification equivalent; the equivalent weight of the ester, which is similar to the neutralization equivalent of an acid.
Reduction of Esters
i) Catalytic hydrogenation:

ii) Chemical reduction is carried out by use of sodium metal and alcohol, or more usually by use of lithium aluminium hydride.
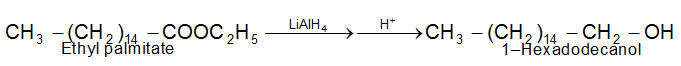
Claisen Condensation
When ethyl acetate reacts with sodium ethoxide, it undergoes a condensation reaction. After acidification, the product is a b - keto ester, ethyl aceto acetate (commonly known as – aceto acetic ester)
Condensation of this type is known as Claisen Condensation. For esters, it is the exact counterpart of the Aldol Condensation. Like the Aldol Condensation, the Claisen Condensation involves nucleophilic attack by a carbanion on an electron – deficient carbonyl compound. In the Aldol Condensation, nuclephilic attack leads to addition (the typical reaction of aldehydes and ketones). In the Claisen Condensation, nucleophilic attack leads to substitution (the typical reaction of acyl compounds)
Mechanism of Claisen Condensation

This step is highly favourable and draws the overall equilibrium toward the product formation
When planning a claisen condensation with an ester it is important to use alkoxide ion that has the same alkyl group as the alkoxyl group of the ester. This is to avoid the possibility of transesterification.
An intramolecular claisen condensation is called Dieckmann condensation.
In general, the Dieckmann condensation is useful only for the preparation of five and six membered rings.
Acid Chlorides
Acid chlorides are prepared from the corresponding acids by reaction with thionyl chloride or phosphorus pentachloride,
Acid chlorides are the most reactive of the derivatives of carboxylic acids.
RCOOH +PCl5 → RCOCl + POCl3 +HCl
RCOOH +PCl3 → 3RCOCl + H3PO3
RCOOH +SOCl2 → RCOCl + SO2 + HCl
Acyl halides are more reactive than alkyl halides in nucleophilic substitution because nucleophilic attack on the tetrahedral carbon of RX involves a hindered transition state. Also, to permit the attachment of the nucleophile a bond must be partly broken. In CH3COCl, the nucleophile, attack on > C = O involves a relatively unhindered transition of acyl halides occurs in two steps. The first step is similar to addition to carbonyl compound and the second involves the loss of chlorine in this case.
Acylation
Acid Chlorides are important acylating agents for compounds having –OH, –SH, –NH2 and –NHR group. During acylation, hydrogen atom of these group is replaced by RCO–group. Acetylation is an example of acylation and is carried out by acetyl chloride.
CH3COCl + HOH → CH3COOH + HCl
Acetyl Chloride Acetic Acid
CH3COCl + HOC2H5 → CH3COOH + HCl
Acetyl Chloride Ethyl Acetate
Reaction of Acetyl Chloride with Olefins
Acetyl chlorides add on to the double bond of an olefin in the presence of a catalyst e.g., zinc chloride or aluminium chloride, to form a chloro ketone which, on heating, eliminates a molecule of hydrogen chloride to form an unsaturated ketone.
Conversion of Acid Chlorides into Acid Derivatives
Amides and esters are usually prepared from the acid itself. Both the preparation of the acid chloride and its reaction with ammonia or an alcohol are rapid, essentially irreversible reactions.
Note: Formyl chloride is present in the form of carbon monoxide and hydrogen chloride at ordinary temperature.
Amides
In the laboratory amides are prepared by the reaction of ammonia with acid chlorides or acid anhydrides. In industry they are often made by heating the ammonium salts of carboxylic acids.
Hydrolysis of Amides
It involves nucleophilic substitution, in which the NH2 group is replaced by –OH. Under acidic conditions hydrolysis involves attack by water on the protonated amide.
Under alkaline conditions hydrolysis involves attack by the strongly nucleophilic hydroxide ion on the amide itself.
Amides are very feebly basic and form unstable salts with strong inorganic acids. e.g. RCONH2.HCl. The structure of these salts may be I or II
Acidic Character of Amides:
Amides are also feebly acidic; e.g. they dissolve mercuric oxide to form covalent mercury compound in which the mercury is probably linked to the nitrogen.
2RCONH2 + HgO→ (RCONH)2Hg + H2O
Reduction of Amides
Amides are reduced by sodium ethanol, catalytically, or by lithium aluminium hydride to a primary amine.

Reaction of Amides with Phosphorus Pentoxide
When heated with P2O5, amides are dehydrated to alkyl cyanides.

Alkyl cyanides are also formed when the amides of the higher fatty acids are heated in the presence of ammonia.

Amides may also be converted into cyanides by phosphorus pentachloride.
Reaction of Amides with Nitrous Acid
When amides are treated with nitrous acid, nitrogen is evolved and the acid is formed.
RCONH2 + HNO2 → RCO2H + N2 + H2O
You can also refer to Organic Chemistry Revision Notes and IIT JEE Chemistry Syllabus