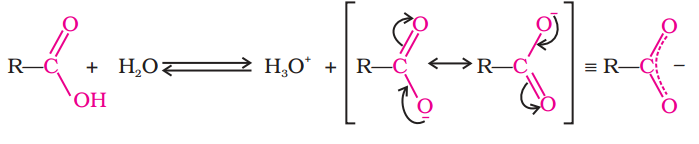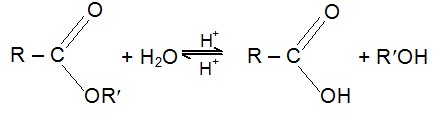Chemical Properties of Carboxylic Acids
Table of Content |
The characteristic chemical behavior of carboxylic acids is, of course, determined by their functional group, carboxyl, –COOH. This group is made up of a carbonyl group (C = O) and a hydroxyl group (–OH). As we shall see, it is the –OH that actually undergoes nearly every reaction. Loss of H+, or replacement by another group but it does so in a way that is possible only because of the effect of the C = O.
Acidity of Carboxylic Acids
Carboxylic acids are weak acids and their carboxylic anions are strong conjugate bases are slightly alkaline due to the hydrolysis of carboxylate anion compared to other species, the order of acidity and basicity or corresponding conjugate bases are as follows:
Acidity RCOOH > HOH > ROH > HC CH > NH3 > RH
CH > NH3 > RH
Basicity RCOO– < HO– < RO– < HCC– < NH2-< R–
Reaction of Carboxylic Acids with Metals
The carboxylic acids react with metals to liberate hydrogen and are soluble in both NaOH and NaHCO3 solutions. For example,
2CH3COOH + 2Na → 2CH3COO–Na+ + H2
CH3COOH + NaOH → CH3COO–Na+ + H2O
CH3COOH + NaHCO3 → CH3COO–Na+ + H2O + CO2
The carboxylic acids react with metals to liberate hydrogen and are soluble in both NaOH and NaHCO3 solutions. For Example
Carboxylic acids dissociate in water to give resonance stabilised carboxylate anions and hydronium ion.
Ionization of Carboxylic Acids, Acidity Constant
In aqueous solution, the carboxylic acids undergo self ionization and exist in equilibrium with the carboxilate anion and the hydrogen ion or hydronium ion.
RCOOH + H2O  RCOO- + H3O+
RCOO- + H3O+
The equilibrium constant for the reaction would be
Keq = [ RCOO-][ H3O+]/[H2O][RCOOH]
Here water is solvent, so its concentration would remain almost same throughout the reaction.
so, Keq = [ RCOO-][ H3O+]/[RCOOH]
Ka = Keq[H2O]
Where Keq, is equilibrium constant and Ka is the acid dissociation constant.
For convenience, the strength of an acid is generally indicated by its pKa value rather than its Ka value.
pKa = – log Ka
-
Smaller the pKa, the stronger the acid . Strong acids have pKa values < 1,
-
The acids with pKa values between 1 and 5 are considered to be moderately strong acids,
-
Weak acids have pKa values between 5 and 15,
-
Extremely weak acids have pKa values >15.
Carboxylic acids are weaker than mineral acids, but stronger acids than alcohols and many simple phenols
Effect of substituents on the acidity of Carboxylic Acids
Any factor that stabilizes the anion more than it stabilizes the acid would increase the acidity of carboxylic acids. While any factor that decreases the stability of anion would decrease the acidity of carboxylic acids. Electron withdrawing groups disperse the negative charge and thus stabilize the anion which results in increase in acidity of the carboxylic acids. Electron donating groups intensify the negative charge and the destabilize the anion which results in decrease in acidity of carboxylic acid.
| Carboxylic Acids | Ka Value |
| HCOOH | 17.7 10-5 10-5 |
| CH3COOH | 1.75 10-5 10-5 |
| ClCH2COOH | 136 10-5 10-5 |
| Cl2CHCOOH | 5530 10-5 10-5 |
| Cl3CCOOH | 23200 10-5 10-5 |
| CH3CH2CH2COOH | 1.52 10-5 10-5 |
| CH3CH2CHClCOOH | 1.52 10-5 10-5 |
From the table it is clear that the electron-withdrowing halogen increase the acidity of carboxylic acids. For example, Chloroacetic acid is about100 times stronger than acetic acid.
Conversion of Carboxylic Acids into functional derivatives
Carboxylici acids can be convered into number of other compounds (known as derivatives of carboxylic acids or simply acid derivatives) by replacement of its –OH group by a Cl, OR or NH2 .
-
Replacement of -OH by -OR formes ester.
-
Replacement of -OH by -NH2 formes amide.
Carboxylic acid can be recovered simply by hydrolysis of the acid derivatives or one can say that the functional derivatives are all readily reconverted into the acid by simple hydrolysis.
Conversion of Carboxylic Acids into Esters (Esterification)
?Carboxylic acids are esterified with alcohols or phenols in the presence of a mineral acid such as concentrated H2SO4 or HCl gas as a catalyst.
This reaction is reversible and the same catalyst, hydrogen ion, that catalyzes the forward reaction i.e. esterification necessarily catalyzes the reverse reaction i.e. hydrolysis.
The equilibrium is particularly unfavorable when phenols (ArOH) are used instead of alcohol; yet if water is removed during the reaction, phenolic esters (RCOOAr] are obtained in high yield.
The presence of bulky group near the site of reaction, whether in alcohol or in the acid, slows down esterification (as well as its reverse, hydrolysis).
Reactivity CH3OH > 1° > 2° > 3°
In esterification HCOOH > CH3COOH > RCH2COOH > R2CHCOOH > R3CCOOH
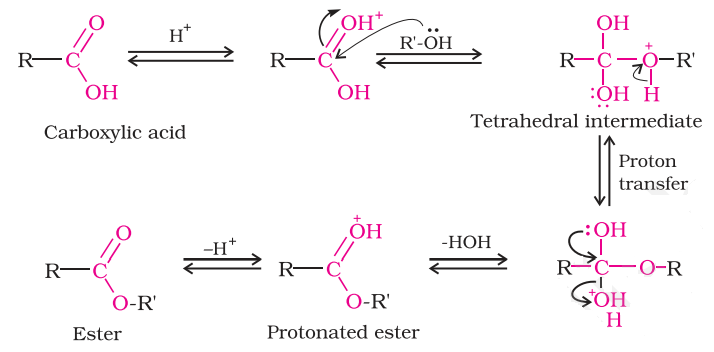
Mechanism of Esterification
The step in the mechanism for the formation of an ester from an acid and an alcohol are the reverse of the steps for the acid-catalyzed hydrolysis of an ester, the reaction can go in either direction depending on the conditions used. A carboxylic acid does not react with an alcohol unless a strong acid is used as a catalyst, protonation makes the carbonyl group more electrophilic and enables it to react with the alcohol, which is a weak nucleophile.
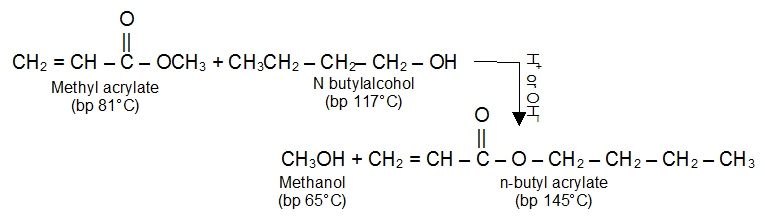
Transesterification
An alcohol is capable of displacing another alcohol from an ester. This alcoholysis (cleavage by an alcohol) of an ester is called transesterification. In other words one can say that it is the process of exchanging the organic group R of an ester with the organic group R’ of an alcohol.
R-OH + R-COOR → R-OH + RCOOR
Alcohol Ester
Transesterification is often catalysed by acid (H2SO4 or dry HCl) or base (usually alkoxide ion). Transesterification is an equilibrium reaction. To shift the equilibrium to the right, it is necessary to use a large excess of the alcohol.
The difference in the boiling points of the alcohols allows the equilibrium to be shifted toward the higher molecular weight ester by distilling the methanol out of the reaction mixture.
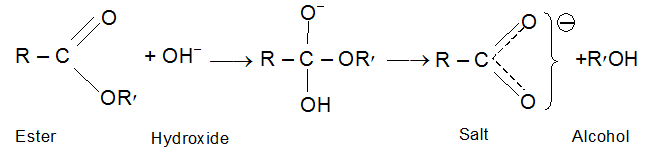
Hydrolysis of esters
Esters are hydrolysed by acids or alkalis
Acidic hydrolysis is reversible and hence the mechanism for hydrolysis is also taken in the opposite direction of the mechanism for esterification.
When hydrolysis is carried out with alkali the carboxylic acid is obtained as its salt. This reaction is essentially irreversible, since a resonance stabilized carboxylate anion show little tendency to react with an alcohol. Since the alkali salts of the higher acids are soaps, alkaline hydrolysis is known as saponification; Saponification is far more rapid than acid hydrolysis. If an ester is hydrolyzed in a known amount of base (taken in excess), the amount of base used up can be measured and used to give the saponification equivalent; the equivalent weight of the ester, which is similar to the neutralization equivalent of an acid.
Reduction of Esters

ii) Chemical reduction is carried out by use of sodium metal and alcohol, or more usually by use of lithium aluminium hydride.
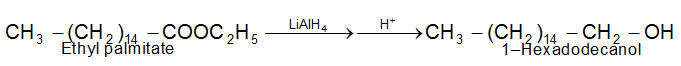
Conversion of Carboxylic Acids into Amides?
?Reaction of carboxylic acids with ammonia results in formation of give ammonium salt which on further heating at high temperature give amides.

Conversion of Carboxylic Acids into Acid Anhydrides
Lower monocarboxylic acid on heating with dehydrating agent (say P2O5) forms anhydrides
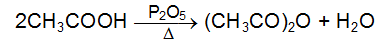
Note: Anhydride of formic acid is not known, it gives CO and H2O on heating with conc. H2SO4.
HCOOH +Conc. H2SO → H2O + CO
Reduction of Carboxylic Acids to Alcohols
Lithium aluminium hydride can reduce an acid to an alcohol; the initial product is an alkoxide from which the alcohol is liberated by hydrolysis.
4 R–COOH + 3LiAlH4 → 4H2 + 2LiAlO2 + (RCH2O)4 AlLi + H2
Hydrolysis of (RCH2O)4 → alcohols
Halogenation of aliphatic carboxylic acids (Hell-Volhard-Zelinsky Reaction)
In the presence of phosphorus, aliphatic carboxylic acids react smoothly with chlorine or bromine to yield a compound in which a-hydrogen has been replaced by halogen.

The function of the phosphorus is ultimately to convert a little of the acid into acid halide so it is the acid halide, not the acid itself, that undergoes this reaction.

The halogen of these halogenated acid undergoes nucleophilic displacement and elimination much as it does in the simple alkyl halides. Halogenation is therefore the first step in the conversion of a carboxylic acid into many important substituted carboxylic acid.
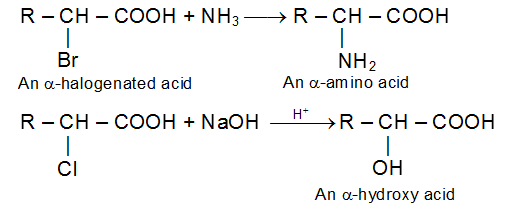
Reaction of Carboxylic Acids with PCl5, PCl3 and SOCl2
The hydroxyl group of carboxylic acids is replaced by chlorine atom on treating with PCl5, PCl3 or SOCl2.
Thionyl chloride (SOCl2) for this reaction because the other two products are gaseous and escape the reaction mixture and we get a pure product
RCOOH +PCl5 → RCOCl + POCl3 +HCl
RCOOH +PCl3 → 3RCOCl + H3PO3
RCOOH +SOCl2 → RCOCl + SO2 + HCl
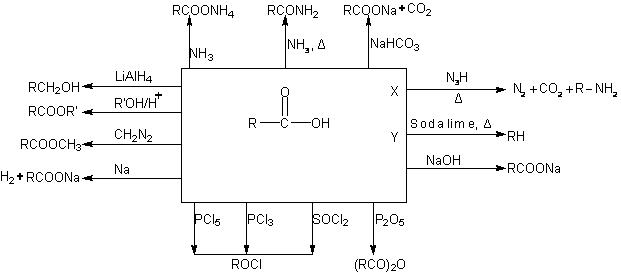
Here is teh summary of all the reactions of carboxylic acids
Refer the below mentioned links to get an immediate solution to all queries on Organic chemistry:
JEE Organic Chemistry Syllabus
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More