Valence Shell Electron Pair Repulsion (VSPER) Theory
Regular Geometry of Molecules
Geometry of the molecules in which the central atom has no lone pairs are regular and can be predicted simply.
Bond ange of any molecule with regular geometry = 360o /Number of bond pairs
Number of electron pairs |
Arrangement of electrons |
Molecular geometry |
Examples |
|
2 |
|
B – A – B Linear |
BeCl2, HgCl2 |
|
3 |
θ = 120° |
θ = 120° |
BF3, AlCl3
|
|
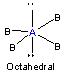
4 |
|
|
CH4, NH4+, SiF4
|
|
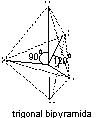
5 |
|
|
PCl5, PF5
|
|
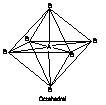
6 |
|
SF6 |
Irregular Geometry of Molecules and VSPER Theory
 Geometry of the molecules having lone pair of electrons can not be predicted simpley using above mentioned method. The geometric arrangement of atoms in molecules and ions may be predicted by means of the valence-shell electron-pair repulsion (VSEPR) theory. This type includes molecules which may or may not obey the octet rule but have only single bonds.
Geometry of the molecules having lone pair of electrons can not be predicted simpley using above mentioned method. The geometric arrangement of atoms in molecules and ions may be predicted by means of the valence-shell electron-pair repulsion (VSEPR) theory. This type includes molecules which may or may not obey the octet rule but have only single bonds.
Postulates of VSEPR theory:
-
The shape of the molecule is determined by repulsions between all of the electron pairs present in the valence shell.
-
A lone pair of electrons takes up more space around the central atom than a bond-pair, since the lone pair is attracted to one nucleus whilst the bond pair is shared by two nuclei. It follows that repulsion between two lone pairs is greater than repulsion between a lone pair and a bond pair, which in turn is greater than the repulsion between two bond pairs. Thus the presence of lone pairs on the central atom causes slight distortion of the bond angles from the ideal shape. If the angle between a lone pair, the central atom and a bond pair is increased, it follows that the actual bond angle between the atoms must be decreased.The descending order of repulsion is
-
(lp – lp) > (lp – bp) > (bp – bp)
-
where lp-Lone pair; bp-bond pair
-
The magnitude of repulsions between bonding pairs of electrons depends on the electronegativity difference between the central atom and the other atoms.
-
Double bonds cause more repulsion than single bonds and triple bonds cause more repulsion than double bonds.
-
A brief summary of molecular shapes resulting from different configurations of electrons pairs is presented below:
-
With very few exceptions, the predictions based on the VSEPR theory have been shown to be correct.
Refer to the following video for geometry of the different molecules
Molecule Type |
No. of Bonding pairs |
No. of lone pair |
Arrangement of electrons pairs |
Shape (Geometry) |
Examples |
|
AB2E |
2 |
1 |
|
Bent |
SO2, O3 |
| AB3E |
3 |
1 |
|
Trigonal pyramidal | NH3 |
| AB2E2 |
2 |
2 |
|
Bent | H2O |
| AB4E |
4 |
1 |
|
See saw | SF4 |
| AB3E2 |
3 |
2 |
T – shaped | CIF3 | |
| AB5E |
5 |
1 |
|
Square pyramidal | BrF5 |
| AB4E2 |
4 |
2 |
Square planar | XeF4 |
To find the shape of a molecule follow the steps given below:
-
Identify the central atom and count the number of valence electrons.
-
Add to this, number of other atoms.
-
If it is an ion, add negative charges and subtract positive charges. Call it total N
-
Divide N by 2 and compare the result with chart I and obtain the shape.
Total N/2 |
Shape of molecule or ion |
Example |
|
2 |
Linear |
HgCl2/BeCl2 |
|
3 |
Triangular planar |
BF3 |
|
3 |
Angular |
SnCl2, NO2 |
|
4 |
Tetrahedral |
CH4, BF4- |
|
4 |
Trigonal Pyramidal |
NH3, PCl3 |
|
4 |
Angular |
H2O |
|
5 |
Trigonal bipyramidal |
PCl5, PF5 |
|
5 |
Irregular tetrahedral |
SF4, IF4+ |
|
5 |
T-shaped |
CIF3, BrF3 |
|
5 |
Linear |
XeF2, I3- |
|
6 |
Octahedral |
SF6, PF6- |
|
6 |
Square Pyramidal |
IF5 |
|
6 |
Square planar |
XeF4, ICI4 |
You can also refer to
To read more, Buy study materials of Chemical Bonding comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More