IIT JEE Equilibrium | JEE Chemical Equilibrium
Table of Content |
 It is an experimental fact that most of the process including chemical reactions, when carried out in a closed vessel, do not go to completion. They proceed to some extent leaving considerable amounts of reactants & products. When such stage is reached in a reaction, it is said that the reaction has attained the state of equilibrium. Equilibrium can be defined as a state at which both the opposite processes take place with equal rate.
It is an experimental fact that most of the process including chemical reactions, when carried out in a closed vessel, do not go to completion. They proceed to some extent leaving considerable amounts of reactants & products. When such stage is reached in a reaction, it is said that the reaction has attained the state of equilibrium. Equilibrium can be defined as a state at which both the opposite processes take place with equal rate.
Equilibrium represents the state of a process in which the properties like temperature, pressure, and concentration etc of the system do not show any change with passage of time. In all processes which attain equilibrium, two opposing processes are involved. Equilibrium is attained when the rates of the two opposing processes become equal. If the opposing processes involve only physical changes, the equilibrium is called Physical Equilibrium. If the opposing processes are chemical reactions, the equilibrium is called Chemical Equilibrium.
We will discuss about chemical and ionic equilibrium involving acid, base and salts in more detail under following topics
Physical Equilibrium
?Physical equilibrium can be defined as the equilibrium in physical processes.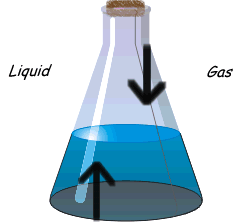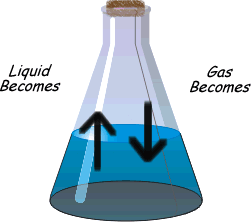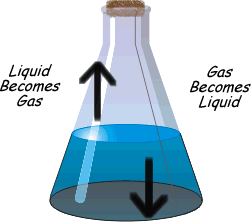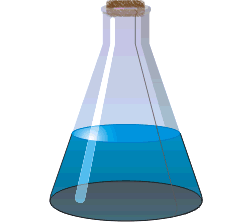 The different types of physical Equilibrium are briefly described below
The different types of physical Equilibrium are briefly described below
(a) Solid – liquid Equilibrium
The equilibrium that exist between ice and water is an example of solid – liquid equilibrium. In a close system, at 0oC ice and water attain equilibrium. At that point rate of melting of ice is equal to rate of freezing of water. The equilibrium is represented as
H2O(s) ⇔ H2O(l)
(b) Liquid – Gas Equilibrium
Evaporation of water in a closed vessel is an example of liquid – gas equilibrium. Where rate of evaporation is equal to rate of condensation. The equilibrium is represented as
H2O(l) ⇔ H2O(g)
(c) Solid – Solution Equilibrium
If you add more and more salt in water taken in a container of a glass and stirred with a glass rod, after dissolving of some amount. You will find out no further salt is going to the solution and it settles down at the bottom. The solution is now said to be saturated and in a state of equilibrium. At this stage, many molecule of salt from the undissolved salt go into the solution (dissolution) and same amount of dissolved salt are deposited back (Precipitation). Thus, at equilibrium rate of dissolution is equal to rate of precipitation.
Salt(Solid) ⇔ Salt(in solution)
(d) Gas –Solution equilibrium
Dissolution of a gas in a liquid under pressure in a closed vessel established a gas – liquid equilibrium. The best example of this type of equilibrium is cold drink bottles. The equilibrium that exists with in the bottle is
CO2(g) ⇔ CO2(in solution)
Equilibrium in Chemical Process (Reversible and Irreversible Reactions)
A reaction in which not only the reactants react to form the products under certain conditions but also the products react to form reactants under the same conditions is called a reversible reaction.
Examples are
-
3Fe(s) + 4H2O(g) ⇔ Fe3O4(s) + 4H2(g)
-
CaCO3(s) ⇔ CaO(s) + CO2(g)
-
N2(g) + 3H2(g) ⇔ 2NH3(g)
If a reaction cannot take place in the reverse direction, i.e. the products formed do not react to give back the reactants under the same condition it is called an irreversible reaction.
Examples are:
-
AgNO3(aq) + NaCl(aq) → AgCl(s) + NaNO3(g)
-
2M(g) + O2(g) → 2MgO(s)
Note: If any of the products will be removed from the system, reversible reaction will become irreversible one.
Generally, a chemical equilibrium is represented as

Where A, B are reactants and C, D are products.
Note:
The double arrow between the left hand part and right hand part shows that changes are taking place in both the directions.
On the basis of extent of reaction, before equilibrium is attained chemical reactions may be classified into three categories.
(a) Those reactions which proceed to almost completion.
(b) Those reactions which proceed to almost only upto little extent.
If all the reactants and products of any reaction under equilibrium are in same physical state, it is called a homogeneous equilibrium?. For example,
N2(g) + 3H2(g)  2NH3 (g)
2NH3 (g)
Here, all the reactants and products are in same phase
Heterogeneous equilibrium? is that type of equilibrium in which ?the state of one or more of the reacting species may differ i.e. all the reactants and products are not in same physical state.
2NaHCO3(s)  Na2CO3(s) + CO2(g) + H2O(l)?
Na2CO3(s) + CO2(g) + H2O(l)?
Here, reactant is solid while in product all the solid, liquid and gaseous matter states are present.
Ionic Equilibrium
Chemical reactions mostly take place in solutions. Solution chemistry plays a very significant role in chemistry. All chemical substances are made up of either polar units (called ions) or non-polar units. The activity of these entities is more evident and pronounced in solution. The behaviour of these substances depends upon their nature and conditions of the medium in which they are added. It is therefore necessary to understand the principles that govern their behaviour in solution.
This type of equilibrium is observed in substances that undergo ionization easily, or in polar substances in which ionization can be induced. Ionic and polar substances are more easily soluble in polar solvents because of the ease of ionization taking place in the solvent medium. With the dissolution of ionic and polar substances in the solvent, these solutions become rich in mobile charge carriers (ions) and thus can conduct electricity. Substances, which are capable of conducting electricity are called as electrolytes while those substances which are non-conducting are called as non-electrolytes.
Characteristics of Equilibrium State
i) It can be attained only if the reversible reaction is carried out in closed vessel.
ii) It can be attained from either side of the reaction.
iii) A catalyst can hasten the approach of equilibrium but does not alter the state of equilibrium.
iv) It is dynamic in nature i.e. reaction does not stop but both forward and backward reactions take place at equal rate.
v) Change of pressure, concentration or temperature favours one of the reactions (forward or backward) resulting in shift of equilibrium point in one direction.
Related Resources
-
Click here for the Detailed Syllabus of IIT JEE Chemistry
-
Look into the Sample Papers of Previous Years
-
You can get the knowledge of Useful Books of Chemistry
To read more, Buy study materials of Chemical Equilibrium comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free
