Ethers
Table of Content |
Physical Properties of Ethers
Since the C–O–C bond angle is not 180o, the dipole moments of the two C–O bonds do not cancel each other; consequently, ethers possess a small net dipole moment (e.g., 1.18 D for ethyl ether).
This weak polarity does not appreciably affect the boiling points of ethers, which are about the same as those of alkanes having comparable molecular weights, and much lower than those of
isomeric alcohols. Compare, for example, the boiling points of n-heptane (98o), methyl n-pentyl ether (100o), and n-hexyl alcohol (1570C). The hydrogen bonding that holds alcohol molecules strongly together is not possible for ethers, since they contain bonded only to carbon.On the other hand, ethers show a solubility in water comparable to that of the alcohols, both ethyl ether and n-butyl alcohol, for example, being soluble to the extent of about 8 g per 100 g of water. We attribute the water solubility of the lower alcohols to hydrogen bonding between water molecules and alcohol molecules; presumably the water solubility of ether arises in the same way.

Industrial Sources of Ethers. Dehydration of Alcohols
A number of symmetrical ethers containing the lower alkyl groups are prepared on a large scale, chiefly for use as solvents. The most important of these is diethyl ether, the familiar anesthetic and the solvent we use in extractions and in the preparation of Grignard reagents; others include isopropyl ether and n-butyl ether.
These ethers are prepared by reactions of the corresponding alcohols with sulfuric acid. Since a molecule of water is lost for every pair of molecules, the reaction is a kind of dehydration. Dehydration to ethers rather than to alkenes

is controlled by the choice of reaction conditions, For example, ethylene is prepared by heating ethyl alcohol with concentrated sulfuric acid to 180o; diethyl ether is prepared by heating a mixture of ethyl alcohol and concentrated sulfuric acid to 140o, alcohol being continuously added to keep it in excess.
Dehydration is generally limited to the preparation of symmetrical ethers, because, as we might expect, a combination of two alcohols usually yields a mixture of three ethers.
Ether formation by dehydration is an example of nucleophilic substitution, with the protonated alcohol as substrate and a second molecule of alcohol as nucleophile.
On standing in contact with air, most aliphatic ethers are converted slowly into unstable peroxides. Although present in only low concentration, these peroxides are very dangerous, since they can cause violent explosions during the distillation that normally follow extractions with ether.
The presence of peroxides is indicated by formation of a red color when the ether is shaken with an aqueous solution of ferrous ammonium sulfate and potassium thiocyanate; the peroxide oxidizes ferrous ion to ferric ion, which reacts with thiocyanate ion to give the characteristic blood-red color of the complex.
Peroxides can be removed from ethers in a number of ways, including washing with solutions of ferrous ion (which reduces peroxides), or distillation from concentrated H2SO4 (which oxidizes peroxides).
For use in the preparation of Grignard reagents, the ether (usually diethyl) must be free of traces of water and alcohol. This so-called absolute ether can be prepared by distillation of ordinary ether from concentrated H2SO4 (which removes not only water and alcohol but also peroxides), and subsequent storing over metallic sodium.
Preparation of Ethers
The following methods are generally used for the laboratory preparation of ethers.
1. Williamson synthesis
In the laboratory, the Williamson synthesis of ethers can be used to make unsymmetrical ethers as well as symmetrical ethers
In the Williamson synthesis an alkyl halide (or substituted alkyl halide) is allowed to react with a sodium alkoxide.
Heating of alkyl halides with sodium or potassium alkoxide give ethers. This is a good method to get better yield of mixed ethers in comparison to above methods. This reaction obeys SN2 mechanism.
R-ONa + R'I  R-OR' + NaI
R-OR' + NaI
R-ONa + RI  R-OR + NaI
R-OR + NaI
Note : alkyl halides undergo elimination reaction with sodium alkoxide to produce alkenes
(CH3)3CCI  (CH3)2C=CH2 + HCI
(CH3)2C=CH2 + HCI
Therefore to prepare t. alkyl-alkyl ether, one must take an alkyl halide with tertiary alkoxide.
CH3CI + (CH3)3CONa → (CH3)3COCH3 + NaCI
Ethers so obtained are contaminated with traces of water and alcohol. Absolute ethers do not possess traces of water or alcohol and are used in preparation of Grignard reagent. Absolute ethers are obtained by distilling ordinary ether with conc. H2SO4 followed by storing over metallic sodium.
Mechanisam of Williamson synthesis
Reaction involves nucleophilic substitution of alkoxide ion for halide ion; it is strictly analogous to the formation of alcohols by treatment of alkyl halides with aqueous hydroxide.

Since alkoxides and alkyl halides are both prepared from alcohols, the Williamson method ultimately involves the synthesis of an ether from two alcohols.
If we wish to make an unsymmetrical dialkyl ether, we have a choice of two combinations of reagents; one of these is nearly always better than the other. In the preparation of tert-butyl ethyl ether, for example, the following combinations are conceivable :

Which do we choose ? Alkoxides are not only nucleophiles, but also strong bases which tend to react with alkyl halides by elimination, to yield alkenes. Whenever we are trying to carry out nucleophilic substitution, one must be aware of the danger of a competing elimination reaction. The tendency of alkyl halides to undergo elimination is 30 > 20 > 10.
In the above example, the use of the tertiary halide is rejected as it would be expected to yield mostly or all elimination product; hence the other combination is used.
2. Alkoxymercuration-demercuration.

3. By alkyl halides :
Alkyl halides on heating with dry Ag2O (in ether) give ethers.

Note : Both methods (i) & (ii) can be used for the preparation of simple ethers. In case of mixed alcohols or mixed halides, the yield is reduced to 1/3rd part producing all possible ethers.
R-OH + HOR' → ROR' + R-OR + R'-OR'
R-X + Ag2O + R'X → ROR' + ROR + R'-OR'
Formation of Aryl Ethers
Phenols are converted into alkyl aryl ethers by reaction in alkaline solution with alkyl halides. For the preparation of aryl methyl ethers, methyl sulfate, (CH3)2SO4, is frequently used instead of the more expensive methyl halides. For example :

The simplest alkyl aryl ether, methyl phenyl ether, has the special name of anisole. In alkaline solutions a phenol exists as the phenoxide ion which, acting as a nucleophilic reagent, attacks the halide (or the sulfate) and displaces halide ion (or sulfate ion).
ArO- + R-X → ArO-R + X-
ArO- + CH3-OSO3CH3 → ArO-CH3 + -OSO3CH3
This is the familiar Williamson synthesis. It is more conveniently carried out here than when applied to the preparation of dialkyl ethers, where the sodium alkoxides must be prepared by direct action of sodium metal on dry alcohols.
Since phenoxides are prepared from phenols, and since alkyl halides are conveniently prepared from alcohols, alkyl aryl ethers (like dialkyl ethers) are ultimately synthesized from two hydroxy compounds.
Because of their low reactivity toward nucleophilic substitution, aryl halides cannot in general be used in the Williamson synthesis. For the preparation of an alkyl aryl ether we can consider two combinations of reactants, but one combination can usually be rejected out of hand. For example
Chemical Properties of Ethers
Ethers are colourless, sweet smelling, highly volatile, inflammable liquids. Ethers are sparingly soluble in water due to H-bonding.
The boiling points of ethers are much lower than corresponding alcohols as they show no hydrogen bonding within themselves like alcohols.
Vapours of diethyl ether cause unconsciousness on inhaling and thus used as anaesthetic agent.
Ethers having bond angle C-O-C to about 110o and thus dipole moment of two C-O bond does not cancel out each other. Thus ethers are polar but weak polarity exist (μ for diethyl ether = 1.18 D)
Ethers are less reactive than compounds containing functional groups. They neither react with active metals, strong bases nor with reducing & oxidizing agents.
The chemical properties of ethers are due to alkyl gp, lone pair of electrons on oxygen atom and cleavage of C-O bond.
1. Cleavage of Ethers by Acids
Ethers are comparatively unreactive compounds. The ether linkage is quite stable toward bases, oxidizing agents, and reducing agents. In so far as the ether linkage itself is concerned, ethers undergo just one kind of reaction, cleavage by acids :
R-O-R' + HX → R-X + R'-OH R'¾X
Reactivity of HX : HI > HBr > HCl
Cleavage takes place only under quite vigorous conditions : concentrated acids (usually HI or HBr) and high temperatures.
A dialkyl ether yields initially an alkyl halide and an alcohol; the alcohol may react further to form a second mole of alkyl halide. For example :

The oxygen of an ether is basic, like the oxygen of an alcohol. The initial reaction between an ether and an acid is undoubtedly formation of the protonated ether. Cleavage then involves nucleophilic attack by halide ion on this protonated ether, with displacement of the weakly basic alcohol molecule :

Such a reaction occurs much more readily than displacement of the strongly basic alkoxide ion from the neutral ether.

Reaction of a protonated ether with halide ion, like the corresponding reaction of a protonated alcohol, can proceed either by an SN1 mechanism,

depending upon conditions and the structure of the ether. As one might expect, a primary alkyl group tends to undergo SN2 displacement, whereas a tertiary alkyl group tends to undergo SN1 displacement.
2.Reactions due to alkyl group :
(a) Combustion : Ethers are highly inflammable and form explosive mixture with air giving CO2 and water.
C2H5O C2H5 + 6O2 → 4CO2 + 5H2O
(b) Halogenation : The alkyl gp of ether undergoes substitution reaction with chlorine or bromine to give a-halogenated ethers in absence of sunlight. However in presence of sunlight all the hydrogen atoms of ethers are substituted.
CH3CH2OCH2CH3 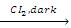 CH3CHCIOCHCICH3
CH3CHCIOCHCICH3
α α'-dichloro diethylether
CH3CH2OCH2CH3 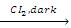 C2CI2OC2CI5
C2CI2OC2CI5
Perchloro diethylether
3. Reaction due to ethereal oxygen :
(a) Basic nature : Due to the presence of two lone pair of electrons on oxygen atom, ethers behave as Lewis bases and form salt with strong acids.

The oxonium salts are soluble in acid solution and regeneration of ether can be made by hydrolysis of these salts.
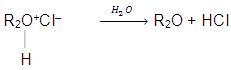
Ethers also form co-ordination complexes with Lewis acids like BF3, AICI3, RMgX etc.

Therefore ethers are very good solvent for Grignard reagents.
4. Formation of Per- oxides :
Ethers form peroxide linkage with oxygen if exposed to air or ozonized oxygen in presence of sunlight or ultraviolet light.

These per oxides are highly poisonous, oily liquids and decompose violenty even at low concentrations. Therefore ethers should not be evaporated to dryness. Also the purity of ether should be checked before its use as anaesthetic agent. An impure ether (having peroxide linkage)- It gives red colour when shaken with ferrous ammonium sulphate and potassium thio-cyanate

On mixing with KI solution liberates I2 which turns starch paper blue.
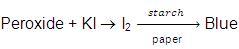
Ethers are made free from peroxide linkage if they are distilled with conc. H2SO4.
Also peroxide formation can be checked if little Cu2O is added to ether.
5.Reactions Involving Cleavage of Carbon-Oxygen Bond :
(a)Action of dil. H2SO4 : Ethers on heating with dilute H2SO4 under pressure are hydrolysed to corresponding alcohols.

(b) Action of Conc. H2SO4 :
Ethers on warming with conc. H2SO4 give alkyl hydrogen sulphate.
R-OR + H2SO4 conc. → 2R HSO4
R-OR' + H2SO4 conc. → RHSO4 + R'HSO4
(c) Action of HI :
(i)The products formed during action of HI on ethers depend upon temperature
R-OR + HI 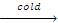 R-OH + RI
R-OH + RI
R-OR' + HI 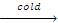 R'-OH + RI
R'-OH + RI
Note : In case of mixed ether, halogen atom gets attached to simpler alkyl gp.
CH3OC2H5 + HI → CH3I + C2H5OH
(ii) R-R + HI 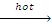 2RI + H2O
2RI + H2O
(iii) Similar reactions are observed with HCI, HBr & the reactivity order if HI > HBr > HCI.
(d) Action of PCI5 :
R-O-R + PCI5 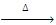 2RCI + POCI3
2RCI + POCI3
(e) Action of Acetyl chloride or Acetic anhydride :
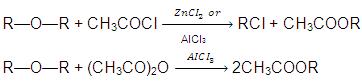
(f) Dehydration of Ethers:
C2H5OC2H5 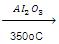 2CH2=CH2 + H2O
2CH2=CH2 + H2O
(g)Action of Carbon Monoxide :
C2H5OC2H5, + CO  C2H5COOC2H5
C2H5COOC2H5
ROR + CO → RCOOR
Uses : Ethers are used as :
(i) General anaesthetic agent
(ii) Refrigerant as produces cooling on evaporation
(iii) Solvent for oils, fats, resins, Grignard reagent etc.
(iv) For providing inert & moisture free medium for reactions e.g. Wurtz reaction.
Click here to access the Organic Chemistry Revision Notes and IIT JEE Chemistry Syllabus
To read more, Buy study materials of Alcohols, Phenols and Ethers comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More



