Alkyl Halides
Table of Content |
|
|
-
Physical Properties of Alkyl Halides
 Because of greater molecular weight, haloalkanes have considerably higher boiling points than alkanes of the same number of carbons. For a given alkyl group, the boiling point increases with increasing atomic weight of the halogen, so that fluoride has the lowest boiling, and iodide the highest boiling point. In spite of polarity alkyl halides are insoluble in water, probably because of their inability to form hydrogen bonds. They are soluble in typical organic solvents.
Because of greater molecular weight, haloalkanes have considerably higher boiling points than alkanes of the same number of carbons. For a given alkyl group, the boiling point increases with increasing atomic weight of the halogen, so that fluoride has the lowest boiling, and iodide the highest boiling point. In spite of polarity alkyl halides are insoluble in water, probably because of their inability to form hydrogen bonds. They are soluble in typical organic solvents.
-
Preparation of Alkyl Halides
1. From Alcohols (Replacement of OH by X)
![R-OH\xrightarrow[or HX]{PX_{3}} R-X](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latexr-oh_xrightarroworhxpx_3r-x.jpg)
Examples :
![CH_{3}CH_{2}CH_{2}OH\xrightarrow[or NaBr, H_{2}SO_{4}, \Delta ]{Conc. HBr} CH_{3}CH_{2}CH_{2}Br](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latexch_3ch_2ch_2oh_xrightarrowornabrh_2so_4_deltaconc.hbrch_3ch_2ch_2br.jpg)



2.Halogenation of Hydrocarbons
![RH \xrightarrow[hv]{X_{2}} RX + HX](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latexrh_xrightarrowhvx_2rxhx.jpg)
Examples

3.Side Chain Halogenation of Alkylbenzenes

4. Addition of Hydrogen Halides to Alkenes

5. Addition of Halogens to Alkenes and Alkynes

6. Halide Exchange

An alkyl iodide is prepared often from the corresponding bromide or chloride by treatment with a solution of sodium iodide in acetone ; the less soluble bromide or sodium chloride precipitates from the solution and can be removed by filtration.A halide ion is an extremely weak base. Its reluctance to share its electrons is shown by its great tendency to release a hydrogen ion, that is, by the high acidity of the hydrogen halides.
When attached to carbon, halogen can be readily displaced as halide ion by other, stronger bases. These bases have an unshared pair of electrons and are seeking a relatively positive site, i.e., are seeking a nucleus with which to share their electrons.Alkyl halides are nearly always prepared from alcohols, which are available commercially or are readily synthesized. Although certain alcohol tend to undergo rearrangement during replacement of -OH by -X, this tendency can be minimized by use of phosphorus halides.Certain halides are best prepared by direct halogenation. The most important of these preparations involve substitution of -X for the unusually reactive allylic or benzylic hydrogens.

Basic, electron rich reagents are called nucleophilic reagents. The typical reaction of alkyl halides is nucleophilic substitution.

-
Reactions of Alkyl Halides
1. Nucleophilic Substitution
|
Reaction |
Product Formed |
|
RX + -OH → ROH + X- |
Alcohol
|
|
RX + H2O→ ROH |
Alcohol |
|
2RX + Na→ R-R + 2NaX |
Alkane (Wurtz reaction) |
|
RX + -OR' → R OR' |
Ether(Williamson synthesis) |
|
RX + -C |
Alkyne |
|
RX + I- → RI |
Alkyl iodide |
|
RX + -CN → RCN |
Nitrile |
|
RX + R'COO- → R'CO-OR |
Ester |
|
RX + :SR' → RSR' |
Thioether (sulfide)
|
|
RX + SH- → RSH |
Thiol (mercaptan) |
|
RX + :NH R'R" → RNR'R'' |
Tertiary amine
|
|
RX + :NH2R' → RNHR' |
Secondary amine |
|
RX + :NH3 → RNH2 |
Primary amine
|
|
RX + ArH + AlCl3 → Ar R |
Alkyl benzene (Friedel Craft reaction)
|
|
|
Malonic ester synthesis |
|
|
Acetoacetic ester synthesis |
Hydrolysis:RX + OH– → ROH + X–
Williamson Synthasis: R-ONa +R'X → R-R' + NaX
Reaction with dry silver oxide: 2R-X + Ag2O → R-O-R
Reaction with sodio-Alkynides: R-C≡C-Na +X-R→ R-C=C-R +NaX
Reaction with potassium-cyanide: KCN+X-R→ RCN +KX
Reaction with silver-cyanide: AgCN+X-R→ RNC +AgX
Reaction with silver-nitrite: AgNO2+X-R→ RNO2 +AgX
Reaction with potassium-nitrite: KNO2+X-R→ R-O-N=O +KX
Fridal Craft Reaction: R-X + C6H6 + AlCl3→C6H5-R
Malonic Ester Synthasis: R-X + -CH(CO2C2H5)2 →R-CH(CO2C2H5)2 +HX
Acetoacetic Ester Synthasis: R-X + -CH(CO2CH3)2 →R-CH(CO2CH3)2 +HX
Reaction with Ammonia: R-X +NH3→ R-NH2 +HX
Wurtz Reaction: 2R-I+ 2Na →R—R + 2NaI
Dehydrohalogenation: CH3.CH2.CH2Br + alco.KOH → CH3–CH = CH2 + KBr + H2O
Reaction with alcoholic AgNO3: R-X +AgNO3 → R+ + AgX↓+HNO3
2. Dehydrohalogenation
Elimination

3. Preparation of Grignard reagent

4. Reduction
RX + M + H+ → RH + M+ + X-
Examples :
CH3)3C Cl  (CH3)3C MgCl
(CH3)3C MgCl  (CH3)3CD
(CH3)3CD
Characteristic Reaction Chart For Alkyl Halides
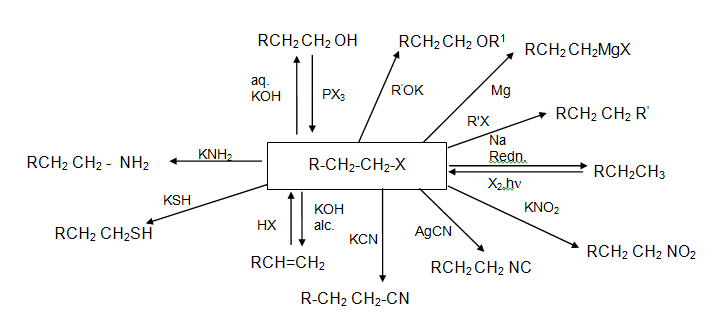
-
Dihalides?
Two H atoms of alkanes are replaced by two halogen atoms to form dihalides. The General formula of dihalides is CnH2nX2.
Dihalides are classified as :
(a)Gem dihalides :
The term gem has derived from geminal meaning for same position.The two similar halogen atoms are attached to same carbon atom e.g.
|
Formula of Gemhalides |
IUPAC Name |
|
CH3CHX2 |
ethylidene dihalide or 1, 1-dihaloehtane |
|
CH3CH2CHX2 |
propylidene dihalide or 1, 1-dihalopropane |
|
(CH3)2CX2 |
isopropylidene dihalide or 2, 2-dihalopropane |
(b) vic dihalides :
The term vic has been derived from vicinal meaning for adjacent carbon atoms.
The two halogens are attached one each on adjacent carbon atoms e.g.
|
Vic dihalides |
IUPAC Name |
|
CH2XCH2X |
ethylene dihalide or 1, 2-dihaloethane |
|
CH3CHXCH2X |
propylene dihalide or 1, 2-diahlopropane |
In these compounds halogen atoms are attached to terminal carbon atoms and are separated by three or more carbon atoms. They are also known as polymethylene halides e.g.
CH2X-CH2-CH2-CH2X tetra methylene dichloride or 1, 4-dihalobutane
(iv) vic and gem diahalides are position isomers to each other.
-
Preparation of Dihalides
(i) By alkenes and alkynes :
CH2=CH2 + X2 → XCH2-CH2X
vicinal dihalide
CH≡CH + 2HX → CH3-CHX2
geminal dihalide

-
Chemical Nature of Dihalides
Lower members are colourless, oily liquids with sweat smell. Higher members are solid.The reactivity of gem dihalides is lesser than alkyl halides, which might be due to the fact that in presence of one halogen atom (with a strong attracting effect i.e.
-I effect), the other cannot be so easily replaced. However vic dihalides have same order of reactivity as alkyl halides.
Thus reactivity order : vic dihalides > gem dihalides. These are heavier than water. The relative density of CH2I2 is 3.325 g/ml which is the 2nd heaviest liquid known after Hg.Some of the important reactions of dihalides are given below.
(a) Action of KOHalc : Both vic and gem dihalides give same products
CH2X - CH2X or CH3-CHX2 + alcoho. KOH → CH≡CH +2HX
b) Acton of Zn dust : Both vic and gem dihalides give same products.
CH2CHX2 or XH2C-CH2X + Zn dust → CH2=CH2
(c) Action of KOHaq
It is a distinction test for gem & vic dihalides.

A gem dihalide gives either aldeyde or ketone on hydrolysis by KOHaq ; A vicinal dihalide gives 1, 2-diol.
(d) Action of KCN followed with hydrolysis and heating the product formed.
A distinction test for geminal and vicinal dihalides.
A geminal dihalide gives acid whereas vicinal dihalide gives anhydride if subjected to action of KCN followed with hydrolysis & heating the product formed.

-CN gp on acid hydrolysis always converts to -COOH.
Two -COOH gp on one carbon atom on heating always lose CO2 to form mono carboxylic acid.
Two -COOH gp on vicinal carbon atom, on heating always lose H2O to form anhydride of acid.
-
General Methods of Preparation of Trihalides
(i) The trihalogen derivative of alkanes are prepared by replacement of three hydrogen atoms by three halogen atoms.
(ii) Their general formula is CnH2n-1 X3 :
(iii) The trihalogen derivative of methane are also known as haloform
- Haloform or trihalo methane CHX3 vis a vis chloroform (CHCI3)
Preparation :
(i) Lab method and Industrial method : By the distillation of ethyl alcohol or acetone with bleaching Powder :
Bleaching powder on hydrolysis gives slaked lime and CI2 which acts as oxidizing agent as well as chlorinating agent.
CaOCI2 + H2O → Ca(OH)2 + CI2
By ethanol :
CI2 + CH3CH2OH → CH3CHO + 2HCI (CI2 acts as oxidant)
CH3CHO + 3CI2 → CCI3CHO + 3HCI (CI2 acts as substituent)
2CCI3CHO + Ca(OH)2 → 2CHCI3 + (HCOO)2Ca (hydrolysis)
chloroform Ca formate
By acetone :
CH3COCH3 + 3CI2 → CCI3COCH3 + 3HCI (CI2 acts as substituent)
2 CCI3COCH3 + Ca(OH)2 → 2 CHCI3 + (CH3COO)2Ca (hydrolysis)
Chloroform is collected with water in lower layer. It is washed with dilute alkali and dried over CaCI2 and then redistilled at 60-65oC.The yield is better if acetone is used.
(ii) By haloform reaction :
Acetaldehyde and all methyl ketones (2-ones) or carbonyl compounds having CH3CO- units as well as alcohols [primary (only ethanol) and secondary (only 2-ol) which produce this unit on oxidation by halogen undergo haloform reaction on heating with halogen and NaOH to give haloform.
If I2 + NaOH is used, the haloform reaction yields yellow solid as precipitate confirming the presence of CH3CO- unit or CH3CH-OH in the molecule attached to C or H.
This is known as iodoform test for confirming the presence of acetaldehyde, methyl ketones and alcohols (which produce of CH3CO-unit on oxidation) e.g.
Reactants which give iodoform test because of CH3CO- unit or producing this unit during oxidation :

In general, acetaldehyde, 2-one, ethanol & sec alcohols (2-ol) give the idoform test. Also pyruvic acid CH3COCOOH, lactic acid CH3CHOHCOOH and acetophenone C6H5COCH3 give this test.The reactions are :
By ethanol
CH3CH2OH + X2 → CH3CHO + 2HX
CH3CHO + 3X2 → CX3CHO + 3HX
CX3CHO + NaOH → CHX3 + HCOONa
5HX + 5NaOH → 5NaX + H2O
CH3CH2OH + 4X2 + 6 → CHX3 + HCOONa + 5NaX + 5H2O
By ethanal :
CH3CHO + 3X2 → CX3CHO + 3HX
CX3CHO + NaOH → CHX3 + HCOONa
3HX + 3NaOH → 3NaX + 3H2O
CH3CHO + 3X2 + 4NaOH → CHX3 + HCOONa + 3NaX + 3H2O
Note :
(a) Ethyl acetoacetate (CH3COCH2COOC2H5) does not give iodoform test, although it contains (CH3CO-gp) attached to carbon (methylene gp). This is due to active nature of methylene group at which iodination occurs and not on the methyl group of (CH3CO-) unit
(b) Certain quinines, quinols and m-dihydric phenols also give positive iodoform test.
(iii) Pure chloroform is obtained by heating chloral hydrate with concentrate sodium hydroxide solution.
CCI3CH(OH)2 + NaOH  CHCI3 + HCOONa + H2O
CHCI3 + HCOONa + H2O
(iv) By chlorination of methane :
CH4 + CI2  CHCI3 + 3HCI
CHCI3 + 3HCI
(v) By CCI4 a commercial method : Commercially it is obtained by the partial reduction of CCI4 with iron fillings and water (steam). This chloroform is not pure and is used only as solvent.
CCI4 + 2H  CHCI3 + HCI
CHCI3 + HCI
Note : Iodoform is commercially obtained by electrolysis of a solution containing ethanol, Na2CO3 and KI. The liberated I2 during electrolysis brings in iodoform reaction with C2H5OH in presence of Na2CO3.
Pure chloroform and bromoform are colourless liquids, and iodoform is yellow solids.
All are heavier than water and soluble in organic solvents, but insoluble in water.
CHCI3 brings temporary unconsciousness when vapours are inhaled for sufficient time and thus used as anaesthetic agent.
CHCI3 is non inflammable but like other halides its vapours when ignited on Cu wire burn with green edge flame. (Beilstein test).
-
Chemical Nature of Trihalides
1. Oxidation :
On exposure to air and sunlight, chloroform is slowly oxidized to a poisonous gas carbonyl chloride i.e. phoszene

Therefore purity of chloroform should be checked before its use as anaesthetic agent. Pure CHCI3 does not give white ppt. of AgCI with AgNO3. Also pure chloroform neither turns blue litmus to red nor give black colour on shaking with H2SO4 conc.
Also following precautions are necessary to store CHCI3 to be used as anaesthetic agent -
It is kept in brown or blue coloured bottles which are filled upto the brim in order to protect the action of light & air.
1% ethanol is also added which acts as negative catalyst for oxidation of CHCI3as well as converts carbonyl chloride into harmless ethyl carbonate.
2. Reduction :
CHCI3 + 2H  CH2CI2 + HCI
CH2CI2 + HCI
CHCI3 + 4H  CH2CI2 + 2HCI
CH2CI2 + 2HCI
CHCI3 + 6H  CH4 + 3HCI
CH4 + 3HCI
3. Hydrolysis or action of KOHaq :
CHCI3 + 4KOH → HCOOK + 3KCI + 2H2O
4. Chlorination :
CHCI3 + CI2  CCI4 + HCI
CCI4 + HCI
5. Action of Ag powder :
![CHX_{2} +6Ag +X_{3}HC \xrightarrow[High temp.]{\bigtriangleup }CH\equiv CH + 6AgX](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latexchx_26agx_3hc_xrightarrowhightemp._bigtriangleupch_equivch6agx.jpg)
6. Action of HNO3 : The H atom of CHCI3 is replaced by -NO2 gp if heated with conc. HNO3.

7. Condensation with acetone :

8. Reaction with sodium ethoxide

9. Riemer - Tiemann reacton : (see phenol)

10. Carbylamine reaction :
All primary amines (may be aliphatic R-NH2 or aromatic Ar-NH2) on warming with CHCI3 and KOHalc. undergo carbylamne reaction to give an offensive or unpleasant odour of isonitrile or carbylamines.

Uses :
- CHCI3, an anaesthetic agent; CHI3 as antiseptic due to liberation of I2.
- CHCI3 as solvent for fat, waxes rubbers, resins etc.
- In preparation of chlorotone (drug, a hypnotic agent) and nitrochloroform (an insecticide).
- CHCI3 as preservative for anatomical specimens
- As laboratory reagent to identify primary amines & other analytical tests.
Note :
- Iodoform on heating with AgNO3 gives yellow ppt. of AgI whereas chloroform does not give this test because of stable nature.
- Iodoform has antiseptic properties because on coming in contact with organic matter of skin it decomposes to give free iodine which acts as an antiseptic.
- Halothane, CF3-CHCIBr, is used as a general anaesthetic which has replaced diethyl ether.
-
Methods of Preparation of Tetrachloromethane
Tetrachloromethane (CCI4)
Manufacture :
(i) From methane : Chlorination of methane with excess of chlorine at 400oC yields impure carbon tetrachloride.

Methane used in this process is obtained from natural gas.
(ii)From carbon disulphide : Chlorine reacts with carbon disulphide in presence of catalysts like iron, iodine, aluminium chloride or antimony pentachloride.
CS2 + 3CI2 → CCI4 + S2CI2
S2CI2 further reacts with CS2 to form more of carbon tetrachloride.
CS2 + 2S2CI2 → CCI4 + 6S
CCI4 is washed with NaOH solution and then distilled to get pure sample.
(iii)By the action of chlorine on chloroform.
CHCI3 + CI2 → CCI4 + HCI
-
Chemical Nature of Tetrachloromethane
Properties :
It is colourless, non inflammable, poisonous liquid with characteristic smell.Insoluble in water but soluble in ethanol and ether. It is a good solvent for oils, fats and greases.
Chemical nature : Less reactive than other halogen derivatives.
1.Oxidation or reaction with steam :

2. Reduction : most iron fillings reduce CCI4 to CHCI3
![CCl_{4}+2[H]\overset{Fe/H_{2}O}{\rightarrow}CHCl_{3}+HCl](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latexccl_42h_oversetfeh_2o_rightarrowchcl_3hcl.jpg)
3.Hydrolysis (action of aqueous KOH) :
CCI4 + 6KOH(aq.) → 4KCI + K2CO3 + 3H2O
Freon-12 (dichlorodifluoromethane) is widely used as a refrigerant and propellant in aerosol sprays of all kinds.
4. Action of hydrogen fluoride in the presence of SbF5.
CCI4 + 2HF  CCI3F2 + 2HCI
CCI3F2 + 2HCI
5. Reaction with phenol and alkali (Reimer-Tiemann reaction).

Uses : Carbon tetrachloride is used
- as fire extinguisher under the name pyrene. Then dense, non combustile vapours over the burning substances and prevents oxygen from reaching them. However, since CCI4 forms phosgene, after the use of pyrene to extinguisher a fire the room should be well ventilated.
- as a laboratory reagent
- as a solvent for oils, fats, resins iodine and in dry-cleaning.
- as a fumigant
- in medicine for the elimination of hookworms as helmenthicide due to antihelminthic nature.
-
Some Useful Halogen Derivatives
1.Freons : The chloro fluoro derivatives of methane and ethane are called freons. Some of the derivatives are : CHF2CI (monochlorodifluoromethane), CF2CI2 (dichloro difluro methane), HCF2CHCI2 (1, 1-dichloro 2, 2-difluoroethane). These are non-inflammable, colourless, non-toxic and low boiling liquids. These are stable upto 550oC. The most important and useful derivative is CF2CI2 which is commonly known as Freon on Freon-12.
Freon or freon-12 (CF2CI2) is prepared by treating carbon tetrachloride with antimony trifluoride in the presence of antimony penta chloride (a catalyst).
3CCI4 + 2SbF3 ![\xrightarrow[catalyst]{SbCl_{5}}](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_xrightarrowcatalystsbcl_5.jpg) 3CCI2F2 + 2SbCI3
3CCI2F2 + 2SbCI3
or by reacting carbon tetrachloride with hydrofluoric acid in presence of antimony penta fluoride.
CCI4 + 2HF ![\xrightarrow[catalyst]{SbCl_{5}}](https://files.askiitians.com/cdn1/cms-content/common/latex.codecogs.comgif.latex_xrightarrowcatalystsbcl_5.jpg) CCI2F2 + 2HCI
CCI2F2 + 2HCI
Under normal conditions Freon is a gas. (b.pt. - 29.8oC). It can easily be liquefied. It is chemically inert and is used in air-conditioning and in domestic refrigerators.
Note : Freon-14 is CF4, Freon-13 is CF3CI, Freon-11 is CFCI3. All these are used as refrigerant.
2. Teflon : A plastic like substance produced by the polymerization of tetrafluoroethylene (CF2=CF2). Tetrafluoroethylene is formed when chloroform is treated with antimony trifluoride and hydrofluoric acid.
On polymerization, tetrafluoro ethylene forms a plastic-like material which is called teflon.
nCF2=CF2 → (CF2-CF2)n
tetrafluoro ethylene Teflon
Teflon is chemically inert substances. It is not affected by strong acids and even by boiling aquaregia. It is stable at high temperature and thus, used for electrical insulation and preparation of gasket materials.
3.Acetylene tetrachloride (Westron), CHCI2CHCI2 :
Acetylene tetrachloride is also known as sym. tetrachloroethane. It is prepared by the action of chlorine on acetylene in presence of a catalyst such as ferric chloride, aluminium chloride, iron, quartz or kieselguhr.
CH≡CH + 2CI2 → CHCI2CHCI2
In absence of catalyst, the reaction between chlorine and acetylene is highly explosive producing carbon and HCI. The reaction is less violent in presence of catalyst.
It is a heavy, non-inflammable toxic liquid with smell like CHCI3. It is insoluble in water but soluble in organic solvents.
On further chlorination, it forms penta and hexachloroethane. On heating with lime (calcium hydroxide), it is converted to a useful product westrosol (CCI2=CHCI)
2C2H2CI4 + Ca(OH)2 → 2CHCI2=CCI2 + CaCI2 + 2H2O
Both westron and westrosol are used as solvent for oils, fats and varnishes.
Look here for Organic Chemistry Revision Notes, Chemistry Syllabus and Best books of Organic Chemistry.
To read more, Buy study materials of Halo Alkanes & Halo Arene comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free
 CR' → R-CºCR'
CR' → R-CºCR'
