General Properties of the Transition Elements (d-Block)
Table of Content |
Introduction to General Properties of the Transition Elements
The elements with incompletely filled d-subshell in their ground state or most stable oxidation state are named as D-block elements. They are additionally named as transition elements. The partially filled subshells incorporate the (n-1) d subshell. All the d-block elements have a similar number of electrons in the furthest shell. Consequently, they indicate comparable chemical properties.
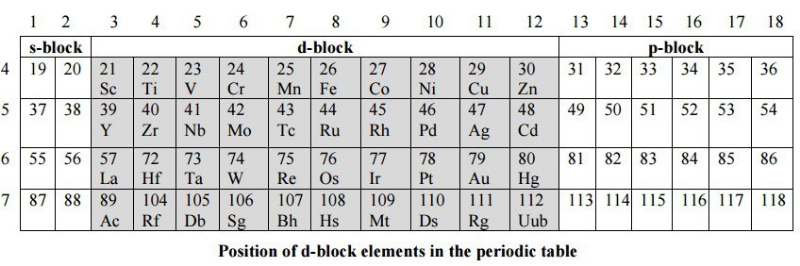
Position of Transition Elements in Periodic Table
The transition elements are set amongst S and P block elements in the periodic table. They begin from the fourth period of the long type of periodic table.
The transition elements can be either non-typical transition elements or the typical transition elements. Elements of II B group Zn, Cd and Hg have a totally filled (n-1) d subshell and subsequently, can't be incorporated into the D-block. Be that as it may, they indicate properties, for example, development of complexes with ligands, for example, amines, ammonia, and halide particles and are consequently, examined alongside d-block elements. Also, the elements of group III-A Sc, Y, La and Ac contrast from other d-block elements, however, have a not entirely filled (n-1)d subshell and are consequently, contemplated alongside d-block elements.
Fig. 1: Position of d- block elements in the periodic table
These elements i.e. elements of group II- B Zn, Cd and Hg and III- A Sc, Y, La, and Ac are named as non-typical transition elements and the other transition elements are named as typical transition elements.
The d-block incorporates three series each of ten elements. These series are portrayed by the totally filled 3d, 4d and 5d subshells and are named as 3d-(first series) Sc - Zn, 4d series (second series) Y-Cd and the 5d series (third series) La-Hg separately. There is a fragmented fourth series comprising of just three elements specifically Ac, Ku, and Ha. In these elements, the 6d subshell begins to fill at Ac.
General Characteristics of D-Block elements
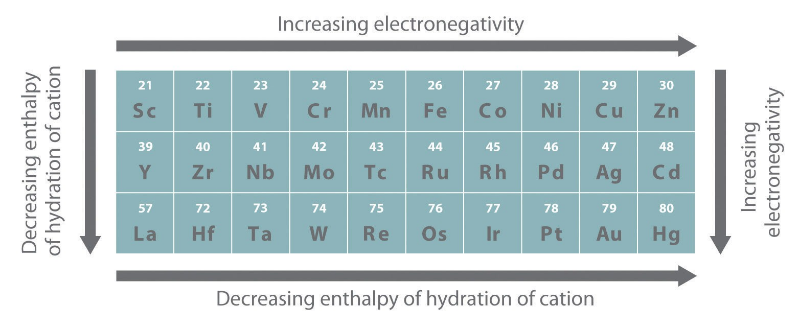
Every one of the elements of the D-block demonstrates comparable properties because of the nearness of comparable electronic configuration of the peripheral shell. The peripheral shell configuration is ns2. Here is a rundown of general properties, for example, the nuclear and ionic radii, electronic configuration, and the ionization potentials seen among the D-block elements.
Fig. 3: General Periodic Properties of D- block Elements
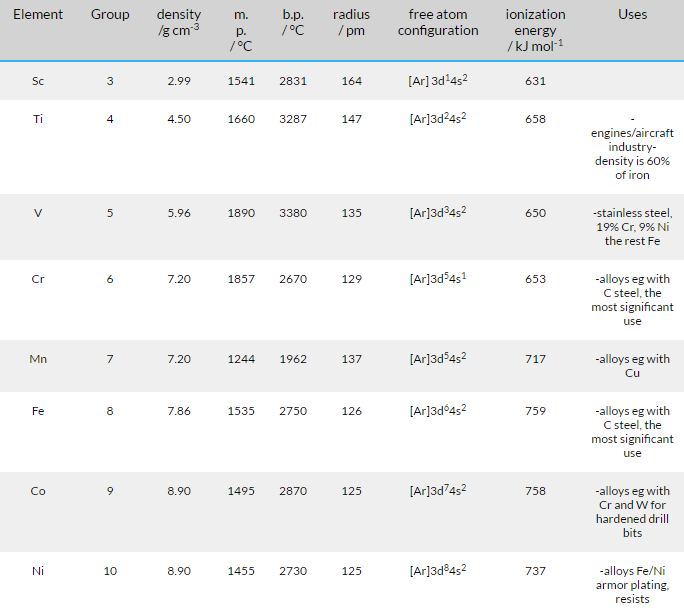
Fig. 4: Properties of the first transition series of Elements
Metallic Nature
Since the number of electrons in the peripheral shell is less, all the transition elements are metals. They demonstrate the qualities of metals, for example, ductility and malleability in nature and shape alloys with a few different metals. They likewise serve as great conductors of electricity and heat. Aside from Mercury which is fluid and delicate like alkali metals all the transition elements are hard and fragile, not at all like the non-transition elements. The hard and fragile nature of these elements demonstrates the nearness of covalent bond which is because of the nearness of unfilled d-orbitals. Be that as it may, their property, for example, great conductivity is a sign for the existence of metallic bonding. Thus, they are said to frame covalent bonding and the metallic bonding.
Melting and Boiling Points
They indicate high melting and boiling points. This can be credited to the nearness of solid metallic bonding because of the overlapping of (n-1)d orbitals and covalent bonding of the unpaired d orbital electrons. Since Zn, Cd and Hg have totally filled (n-1)d orbitals they are not anticipated that they would frame covalent bonds. Thus, they demonstrate lower melting point as compared to other d-block elements.
Atomic Radii
An extraordinary variation is found in the nuclear radii over every transition series. The nuclear radii of the d-block elements inside a given series decrease with increment in the nuclear number. This is because of the expansion in the atomic charge that attracts the electron cloud inwards bringing about a decrease in size. Notwithstanding, a uniform reduction in nuclear radius is not seen over a period. The abatement in nuclear radii is small as contrasted with the S and P block elements. This is because of the screening impact brought about by the electrons of the (n-1)d subshell on the peripheral shell. Subsequently, the nucleus can't pull the furthest electrons. Consequently, the size of the particle does not modify much in moving from Cr to Cu.
The nuclear radius increases as we move down the group. In a given series, the nuclear radius declines to the lowest value for the group VIII elements and after that, it increments towards the end of the series. This expansion in radius towards the finish of the series is because of the force of repulsion among the additional electrons. A close similitude is seen in the radii of the elements of the second and third transition series because of the filling of 4f subshells.
The nuclear size or ionic radii of tri-positive lanthanide particles demonstrate a stable and progressive decline with the expansion in nuclear number from La to Lu. In spite of the fact that they demonstrate a few anomalies, the ionic radii diminish relentlessly from La to Lu. This slow decrease in the size with expanding nuclear number is called lanthanide contraction.
Fig. 5: Variation in atomic radius of transition metals as a function of the periodic table group number
Ionic Radii
The pattern of ionic radius is similar to the atomic radii pattern. Hence, for ions of a given charge the radius decreases gradually with an increment in atomic number.
Atomic Volume and Densities
The nuclear volume of transition elements is much lower than those of S and P block elements. This is a result of the filling of the (n-1)d orbitals that cause an expansion in the atomic charge and pulls the electrons internally. This leads to a diminishment in nuclear volume. With the decline in the nuclear volume, the nuclear thickness of these elements increments. Osmium consists of the highest density.
In a given transition series, the thickness increments in moving over the period and achieves the greatest value at group VIII.
The thickness increases as we move down the group. The nuclear sizes of elements of the second and third transition series are approximately same, yet their nuclear weights increment to almost double and the densities of elements of third transition series are by and large twice of the corresponding second transition series.
Ionization Potentials
Transition elements have high ionization energy because of their small size. Their ionization potentials lie between those of S and P block elements. Subsequently, they are less electropositive than the s-block elements. Henceforth, they don't shape ionic compounds promptly as compared to the alkali and alkaline earth metals. They likewise have the capacity to frame covalent compounds.
The ionization potentials of d-block elements increment as we move over every series from left to right. Be that as it may, the expansion is not as much as in the occurrence of S and P blocks elements. This is because of the screening impact created by the new electrons that are included into the (n-1)d subshell.
The second ionization energies of the primary transition series additionally increment with the expansion in nuclear number. Be that as it may, Cr and Cu are adequately higher than those of their neighbors. This is because of their stable electronic configuration.
Electronic Configuration
The external electronic configuration stays consistent. Be that as it may, an electron is added to the penultimate shell till the d-subshell achieves its full limit. There are three series of elements relying upon the n-1 d orbital that is being filled. The orbitals are dispatched altogether of their expanding energy i.e. an orbital of lower energy is filled first. Therefore 4s orbital with lesser energy is filled first to its full degree then the 3d orbital with higher energy is filled. The precisely half-filled and totally filled d-orbitals are extraordinarily stable.
The electronic configuration of the first series can be represented as 1s22s2p6 3s2p6d1-10 4s2
The electronic configuration of the second series can be represented as 1s22s2p6 3s2p6d1-10 4s2p6d1-10 5s2
The electronic configuration of the third series can be represented as 1s22s2p6 3s2p6d1-10 4s2p6d1-10 5s2p6d1-10 6s2
Exceptional Electronic Configuration of:
Ni: [Ar] 4s1 3d9
Pt: [Xe] 6s1 5d9
Pd: [Kr] 4d10 5s0
-
Transition elements also show variable oxidation states, tendency to form complexes, magnetic nature and other properties.
Oxidation State
All the transition elements, aside from the first and last individuals from the series, display various oxidation states. They indicate variable valency in their compounds. Some fundamental oxidation conditions of the primary, second and third transition series elements are given beneath in tables.
Oxidation States of 3d Series
Oxidation state of 4d series
Oxidation State of 5d Series
Cause for Variable Oxidation States
The valence electrons of the transition elements are in (n-1) d and ns orbitals which have a little distinction in energies. Both energy levels can be utilized as a part of bond development.
They demonstrate the +2 oxidation state because of the 2 electrons in ns orbitals when the electrons of (n-1) d stay unaffected.
The higher oxidation state from +3 to +7 is because of the utilization of all 4s and 3d electrons in the transition series of elements. In the excited state, the (n-1) d electrons get to be bonding and give the variable states to the iota. Subsequently, the variable oxidation state is because of the support of both ns and (n-1) d orbitals in bonding.
Some important features of oxidation state of transition metals are:
-
The most basic oxidation condition of 3d series is +2 with the exception of scandium, because of the loss of two ns electrons. This demonstrates d orbitals are more stable than s orbitals after scandium.
-
The ionic bonds are by and large framed in +2 and +3 state while the covalent bonds are shaped in higher oxidation states. Covalent bonds are framed by the sharing of d-electrons. For instance permanganate particle MnO4–, all bonds shaped amongst manganese and oxygen is covalent.
-
The most noteworthy oxidation state increments with expanding nuclear number of element, achieves greatest in the center and after that begins diminishing as appeared in the table. For instance, Iron demonstrates the normal oxidation condition of + 2 and + 3, however ruthenium and osmium in a similar group shape compounds in the +4, + 6 and + 8 oxidation states.
-
The elements in the start of the series display less oxidation state in view of having smaller number of electrons to lose or contribute. The elements amidst the series demonstrate the best number of oxidation. For instance; Mn demonstrates all the oxidation states from +2 to +7. The most elevated oxidation state appeared by any transition metal is eight which is demonstrated by Ru and Os.
-
The inconsistency of oxidation states emerges in a manner that progressive states contrast in unity.
-
The higher oxidation states are more stable in heavier elements. Like in Mo (VI), W (VI) are more stable than Cr (VI).
-
They additionally frame compounds in low oxidation states, for example, +1 and 0 having ligands like CO. For instance, nickel in nickel tetra carbonyl, Ni(CO)4 and Fe in Fe(CO)5 has zero oxidation state. Cu is in +1 state in CuCl.
-
The oxidation condition of a metal in solvents relies upon the solvent's nature. For instance, Cu in +1 state in water is unstable as it might experience oxidation while Cu in +2 states is stable.
-
The oxidation condition of transition metals relies on upon the way of the binding atoms. So the compounds of metal with fluorine and oxygen display the most noteworthy oxidation state because of their little size and the high electro negativity of fluorine and oxygen.
-
The relative stabilities of elements which exist in more than one oxidation state can be known from the standard electrode potential.
-
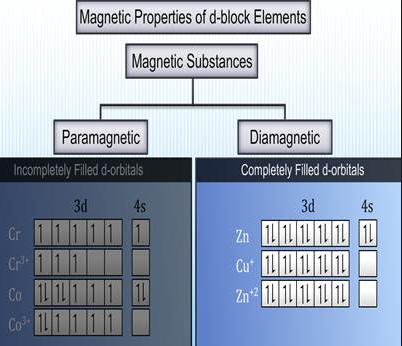
Magnetic Properties:The magnetic properties of D-Block Elements are dictated by the number of unpaired electrons in it. There are two fundamental sorts of substances.
Fig. 6: Magnetic properties of d- block elements
(i) Paramagnetic substances
The paramagnetic character emerges in view of the nearness ofunpaired electrons. Paramagnetic substances are the substances which are pulled in by the magnetic field.
(ii) Diamagnetic Substances
Diamagnetic character emerges as a result of the nonappearance of unpaired electrons. Diamagnetic substances are the substances which are repulsed by the magnetic field.
A large portion of the transition elements and their compounds are paramagnetic and are pulled in by the magnetic field. More prominent the number of unpaired electrons in the substance more noteworthy is the paramagnetic character; the magnetic character of a substance is expressed as magnetic moments. The magnetic moment can be computed utilizing the following relation
μ = √{n(n + 2)} BM (Bohr Magneton)
where, n = number of unpaired electrons
For Example,
Ti3+ – The number of unpaired electrons is 1.
Hence,
μ = √{1(1+ 2)} BM = 3 = 1.732 B.M
Bigger the magnetic moment's value, the more noteworthy is the paramagnetic character.
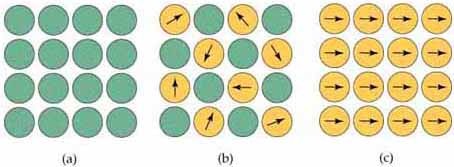
Notwithstanding paramagnetic and diamagnetic substance, there are a couple of substances, for example, iron which is exceedingly magnetic when contrasted with other metals.
These substances are known as Ferromagnetic Substances.
Fig. 7: Types of magnetic behavior: (a) Diamagnetic; no centers (atoms or ions) with magnetic moments. (b) Simple paramagnetic; centers with magnetic moments are not aligned unless the substance is in a magnetic field. (c) Ferromagnetic; coupled centers aligned in a common direction.
- Formation of colored compounds: Most d-block metal compounds are coloured in their solid or liquid states.
In the case of transition metal ions, under the influence of ligands, the degeneracy of the 5 d-orbitals is lost and they separate into two distinct energy levels.
eg set: d x2- y2 and dz2 orbitals
t2g set: dxy, dxz and dyz orbitals
Fig. 8: Formation of colored compounds
When white light is incident on a transition metal ion, the electron in the lower energy d-orbital set absorbs certain radiations and gets promoted to a d-orbital set of higher energy. The transmitted radiation devoid of the absorbed radiations is the complementary colour of the absorbed light. This complementary colour is the colour of the substance.
Patterns to Remember
-
All transition-metal cations have dn electron configurations; the ns electrons are constantly lost before the (n − 1) d electrons.
-
Transition metals are described by the presence of different oxidation states isolated by a solitary electron.
-
Most transition-metal compounds are paramagnetic, while theoretically all compounds of the p-block elements are diamagnetic.
-
The most astounding conceivable oxidation state, relating to the formal loss of all valence electrons, turns out to be progressively less stable as we go from group 3 to group 8, and it is never seen in later groups.
-
In the transition metals, the stability of higher oxidation states increments down a segment.
Summary of selected physical properties of transition elements
Frequently Asked Questions (FAQs)
Q1. What do you mean by d-d transition?
Sol.In the transition metal particles, the electrons can be advanced from one energy level to another energy level in a similar d-subshell. This process is called d-d-transition. By this property just transition elements indicates color.
Q2. What are transition elements and why are they called so?
Sol. The elements which have mostly filled d-orbitals either in ground state or in at least one of their oxidation state are called d-block elements or transition elements. They are called so on the grounds that their properties are intermediate between s-block elements and p-block elements. They are more electropositive than p-block elements however less electropositive than s-block elements. They are all metals.
Q3. What is meant by transition elements?
Sol. Transition elements imply their properties are intermediate between s-block elements and p-block elements. They are more electropositive than p-block elements however less electropositive than s-block elements. They are all metals.
Q4. How reactive are transition elements?
Sol. The transition metals are put in the middle point of the periodic table, between groups 2 and 3. They are for the most part hard and thick, and less reactive than the alkali metals. Iron, copper, silver and gold are vital transition metals.
Watch this Video for more reference
More Readings
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free