Extraction of Copper and Aluminium
Table of Content |
Extraction of Copper
The chief and important ore of copper from which the metal is most isolated is copper pyrites (CuFeS2). Froth floatation process is used for concentrating the ore. The powdered ore is suspended in water and after adding little pine oil is stirred by means of air. The sulphide ore particles come to the surface & gangue remains at the bottom is rejected.
1. Ores of Copper
|
Name of Ore |
Formula of Ore |
|
Copper glance or Chalcocite: |
Cu2S |
|
Chalcopyrites(copper pyrites): |
|
|
Cuprite |
Cu2O |
|
Malachite |
[CuCO3.Cu(OH)2] |
|
Azurite |
[2CuCO3.Cu(OH)2] |
2. Extraction of Metallic Copper
Concentration of the ore by froth floatation process:
 Copper pyrites contains only (2-3)% of copper. The rest of the ore contains iron or sulphide, silica, silicious materials, sulphur, arsenic etc. as impurities. Froth flotation by Xanthate and pine oil. The froth is collected and dried when concentrated ore is obtained which contains 25-30% of Cu.
Copper pyrites contains only (2-3)% of copper. The rest of the ore contains iron or sulphide, silica, silicious materials, sulphur, arsenic etc. as impurities. Froth flotation by Xanthate and pine oil. The froth is collected and dried when concentrated ore is obtained which contains 25-30% of Cu.
Roasting
Cu2S.Fe2S3 + O2 → Cu2S + 2FeS + SO2
Cu2S.Fe2S3 + 4O2 → Cu2S + 2FeO + 3SO2
2Cu2S + 3O2 → 2Cu2O + 2SO2
Cu2O + FeS → Cu2S + FeO
 Smelting of the roasted ore in blast furnace: material required
Smelting of the roasted ore in blast furnace: material required
-
Roasted ore
-
Lime stone
-
Coke (used as fuel)
-
Silica (used as flux)
-
Lime stone (used to remove excess silica)
-
Reactions occurring given as follows
2FeS + 3O2 → 2FeO + 2SO2
Cu2O + FeS → Cu2S + FeO
FeO + SiO2 → FeSiO3 (removed as slag)
CaO + SiO2 → CaSiO3 (removal as slag)
Self reduction in Bessemer Converter
- Reactions involved are,
2FeS + 3O2 → 2FeO + 2SO2
FeO + SiO2 → FeSiO3 (slag)
2Cu2S + 3O2 → 2Cu2O + 2SO2
Cu2S + 2O2 → Cu2SO4
When 2/3 of the cuprous sulphide is oxiidsed, the balst is stopped. The produced Cu2O and Cu2SO4 are reduced by the rest of cuprous sulphide to produce metallic copper with the evolution of SO2.
Cu2S + 2Cu2O → 6Cu + SO2
Cu2SO4 + Cu2S → 4Cu + 2SO2
As the molten copper cools, it gives off the dissolved of SO2. The SO2 gas escaping in the form of bubbles, leaves the surface of the metal with full of cavities which gives the metal a blistered appearnace. This is why the metal thus obtained is by blister copper.
4. Refining of Copper
Copper is refined using electrorefining method.
Anode: Impure copper obtained above
Cathode: Pure copper
Electrolyte: 15% CuSO4 solution + 5% H2SO4
When electric current is passed through the electrolyte, the anodes gradually dissolve and pure copper is deposited on the cathodes which gradually grow in size. The impurities like Fe, Zn, Ni etc., dissolved in the solution as sulphates while gold, silver, platinum settle down below the anode as anode mud.
Reactions coming are as follows
CuSO4
Cu+2 + SO4–2
At anode: Cu – 2e
Cu+2
At cathode: Cu+2 + 2e
Cu
Extraction of Aluminium
 Aluminium is the most abundant metal in the earth’s crust. Aluminium does not occur free in nature, but its compounds are numerous and widely distributed.
Aluminium is the most abundant metal in the earth’s crust. Aluminium does not occur free in nature, but its compounds are numerous and widely distributed.
The chief and important ore from which aluminium is exclusively and profitably obtained is Bauxite, AI2O3.2H2O. The extraction of the metal from bauxite involves the three main steps.
- Purification of Bauxite
- Electrolytic reduction of Alumina, (AI2O3)
- Purification of AI.
1. Ores of Aluminium
|
Name of Ore |
Formula of Ore |
|
Bauxite |
Al2O3×2H2O |
|
Cryolite |
|
|
Feldspar |
K2Oal2O3×6SiO2 or KalSi3O8 |
|
Mica |
K2O×3Al2O3×6SiO2×2H2O |
|
Corundum |
Al2O3 |
Aluminium is mainly extracted from bauxite ore.
2. Extraction of Aluminium
Purification of Bauxite
By Bayer’s process comercially it is being carried out (for red bauxite not for the white bauxite).
Flow sheet of Bayer’s process for the preparation of pure Al2O3
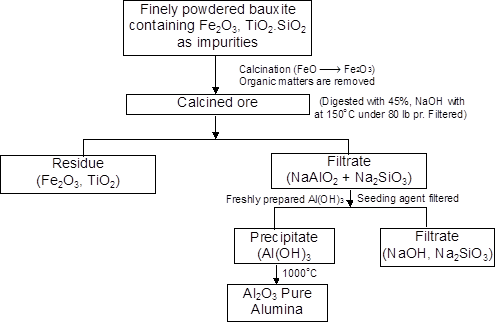
Hall’s process
Crude bauxite at 1100°C reacts with Na2CO3, little CaCO3 when CaSiO3, NaSiO2, NaFeO2 etc. form
Al2O3 + Na2CO3 → 2NaAlO2 + CO2
Fe2O3 + Na2CO3 → 2NaFeO2 + CO2
SiO2 + Na2CO3 → Na2SiO3 + CO2
CaO + SiO2 → CaSiO3
Then at 50° – 60°C CO2is passed through NaAlO2 solution and produces thereby Al(OH)3
2NaAlO2 + CO2 + 3H2O ¾® 2Al(OH)3¯ + Na2CO3
2Al(OH)3
Al2O3 + 3H2O
Serpeck’s Process
Bauxite containing high percentage of silica can be purified by Serpeck’s process. In this process finely powdered bauxite is mixedf with coke and the mixture is heated to 1800°C in a current of nitrogen. The AlN thus obtained is reacted with hot and dilute NaOH, produced NaAlO2 and excess AlN is hydrolysed and Al(OH)3 is formed.
Al2O3 + 3C + N2 → 3AlN + 3CO
SiO2 + 2C → Si + 2CO
AlN +NaOH → NaAlO2 + NH2+
NaAlO2 + 2H2O → Al(OH)3¯ + NaOH
AlN + 3H2O → Al(OH)3¯ + NH3
2Al(OH)3
Al2O3 + 3H2O
Electrolytic Reduction of Al2O3
Pure alumina melts at about 2000°C and is a bad conductor of electricity. If fused cryolite AlF3.3NaF and CaF2 (Fluorspar) is added the mixture melts at 900°C and Al2O3 becomes a good conductor of electricity. Metallic Al is liberated at the cathode

Alumina is mixed with cryolite (Na3AIF3), fluorspar (CaF2) in the ratio 20 : 60 whereby, it not only becomes good conductor but also fuses at about 900oC which is much below the b.p. of aluminium.
The electrolysis of the fused mass is carried out in an iron box, which lined with gas carbon. The lining serves as the cathode, the anode consists of carbon rods dipped in the fused mass. The fused electrolyte is kept covered with a layer of powdered coke to prevent any action of air. The voltage employed in the electrolysis is 5.3 volts. The current passed (about 50,000 amperes) serves to purposes: (i) heating and (ii) electrolysis. Thus the fused mass is automatically kept at 900oC during electrolysis.
Aluminium is obtained at the cathode and being heavier than the electrolyte sinks to the bottom and is tapped off periodically from the tap hole. Oxygen liberated at the anode attacks carbon rods and forms CO and CO2. During electrolysis the concentration of the electrolyte goes on falling thereby increasing the resistance of the cell which is indicated by the glowing of a lamp placed parallel. Much of the alumina is then added and the process is made continuous.
Electrolysis of molten mixture
Cathode: Carbon
Anode: Graphite rods
Electrolyte: 60 parts cryolite + 20 parts fluorspar + 20 parts pure Al2O3
Temperature: 900°C
Reactions
According to the 1st theory the following reaction occurs
Al2O3  2Al+3 + 3O–2
2Al+3 + 3O–2
At cathode : 2Al+3 + 6e → 2Al
At anode : 3O–2 – 6e → 3O2
As cryolite has greater electrochemical stability it does not dissociate. It only increases the dissociation of Al2O3
But the second theory states that, cryolite undergoes electrolytic dissociation first then Al+3 goes to the cathode, produced F2 at anode then reacts with Al2O3 produces AlF3.
AlF3.3NaF  Al+3 + 3Na+ + 6F–
Al+3 + 3Na+ + 6F–
At cathode : Al+3 + 3e → Al
At anode : 6F– – 6e → 3F2
Overall Reaction : Al2O3 + 6F2 → 4AlF3 + 3O2
|
Solved Problem |
|
Question 1: Aluminium is not extracted directly from bauxite, instead, bauxite is first purified to produce pure alumina from which aluminium is extracted by electrolytic reduction – Explain why? Solution Aluminium is not directly extracted from bauxite. This is because of the fact that, bauxite is always associated with impurities like ferric oxide and silica. If bauxite is used directly for the extraction of aluminium, the iron and silica present in if would deposit at the cathode during its electrolytic reduction. The aluminium thus obtained at the cathode, becomes contaminated with iron and silica. As a result, the produced aluminium becomes brittle and is readily attacked by air and water. In moist air copper corrodes to produce a green layer on the surface. Explain Solution: In presence of moist air a thin film of green basic copper carbonate is formed on its surface and hence copper corrodes 2Cu + O2 + H2O + CO2 → CuCO3.Cu(OH)2 |
3. Refining of Aluminium
The aluminium metal obtained by the electrolysis of fused almina is about 99.5% pure. It can be further refined by Hoope’s electrolytic process
Aluminium as produced by the electrolysis of AI2O3 is 90% pure. It can be refined further up to 99.9% purity by Hoope’s process.
The electrolytic cell consists of an iron tank lined with carbon. It is filled with three liquids differing in specific gravity. The upper layer is of pure fused aluminium and serves as cathode.
The bottom layer is that of impure metal in the fused state and serves as anode. The central layer is that of molten mixture of the fluorides of AI, Ba and Na and serves as an electrolyte

On passing electric current, pure aluminium goes to the top layer from the central layer and an equivalent amount of the metal from the bottom layer passes into the central layer. There is thus gradual transference of aluminium from bottom layer to the top and the impurities are left behind. Crude aluminium is added from time to time.
4. Uses of Aluminium
- Aluminium, being very light, is used in householf utensils, aeroplane parts, precision and surgical instruments etc.
- Since it is unattached by nitric acid, is used in chemical plants and also for transporting nitric acid.
- Aluminium foil is used for packing chocolates, cigarettes etc.
- Alums are used as mordents in dyeing and points.
- Mixed with oil, it is used in steam piped and other metal objects.
- It is used as a reducing agent for the production of certain metals such as chromium, iron, manganese etc.
- Alumina is used for making refractory bricks and ultramarine.
5. Alloys of Aluminium
Aluminium forms a number of useful alloys, which are given as follow;
|
Alloy |
Approximate composition |
Uses |
|
(1) Aluminium bronze |
AI 10%, Cu 90% |
For hard, non-corrodible vessels |
|
(2) Duralumin |
AI 95%, Cu3% Mn1% Mg1% |
Aeroplanes and automobile parts |
|
(3) Magnalium |
AI 90%, Mg10% |
Balance beams |
|
(4) Y-alloy |
AI 92.5%, Cu 4%, Ni 2%, Mg 1.5% |
Aeroplan |

Question 1: In Serpek’s process, by product obtained in the purification of bauxite is
(A) Al2O3
(B) N2
(C) NH3
(D) None
Question 2: Which of the following metal is thrown as anode mud during electrolytic refining of copper ?
(A) Zn
(B) Fe
(C) Ag
(D) Ni
Question 3: In the electrorefining, the impure metal is made
(A) Cathode
(B) Anode
(C) Both

|
Q.1 |
Q.2 |
Q.3 |
|
c |
c |
b |
Related Resources
-
Click here for past year papers of IIT JEE
-
Click here to have a lookj at syllabus of IIT JEE
-
You can also refer to refining of metals
To read more, Buy study materials of General Principles & Isolation of Elements comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.