Classification of Polymers
Table of Content |
Polymers are large molecules with high molecular mass. They are formed by joining of small units known as monomers.
For detailed study on Polymers, kindly refer to the content Polymers.
Polymers are classified on different basis and types as explained below:
Classification based on the source of Origin
-
Natural Polymers are those which are found in animals and plants. This includes cellulose, starch, proteins, resins etc.
Fig.1. Structure of cellulose
-
Semi-synthetic Polymers are chemically modified natural polymers such as cellulose nitrate, cellulose derivatives such as rayon etc.
Fig.2. Structure of rayon
-
Synthetic Polymers are man-made polymers. It includes polythene, synthetic fibers, synthetic rayon etc. They are man-made products.
Fig.3. Structure of rubber
Classification based on the Structure of Polymers
-
Linear Polymers are straight chain polymers which are formed when monomers are joined end to end. For example, polythene, polyvinyl chloride etc.
Fig.4. Structure of polythene
-
Branched chain Polymers are linear polymers containing branches. It occurs by replacement of any substituents such as hydrogen atom, monomer subunit etc. For example, low density polythene.
Fig. 5. Low density polythene
-
Network Polymers are formed by combination of bi-functional and tri-functional monomers or cross-linking between different polymer chains. They are formed by the formation of covalent bonds between various linear polymers. For example, bakelite, melamine etc.
Fig.6. 3D structure of network polymers
Classification based on the Mode of Polymerization
-
Addition Polymers are formed by the repeated addition of monomers containing double or triple bonds. When the addition occurs via a same monomeric unit, the monomers formed are known as homo-polymers. When different units of monomers are used, the polymer formed is known as copolymer. For example, nitrile rubber.
Fig.7. Structure of nitrile rubber
-
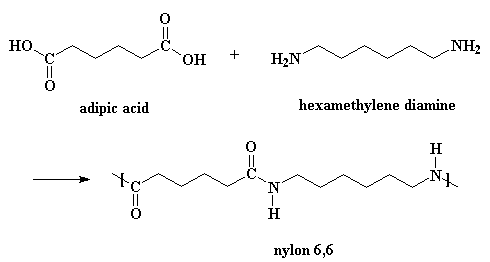
Condensation Polymers are formed by the condensation of different monomeric units. It involves the elimination of water, alcohol, etc. For example, formation of nylon 6,6.
Fig.8. Structure of Nylon-6,6
Classification based on Molecular Forces
-
Fibers are synthetic as well as natural polymers. Synthetic fibers are cheap and easy to made whereas natural fibers are more comfortable for use as compared to synthetic fibers. They possess high tensile strength. They are thread forming solids with strong intermolecular forces such as hydrogen bonds. For example, terylene.
Fig.9. Structure of terylene
-
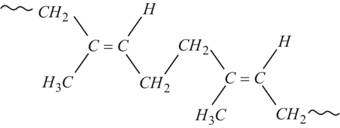
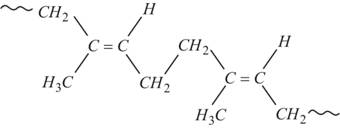
Elastomers are polymers that possess both viscosity and elasticity. For example, rubber, are elastic solids. The polymer is held by weak intermolecular forces. They have the property to retract to its original position after being stretched.
Fig.10. Structure of rubber
-
Thermosetting Polymers are highly cross-linked and branched polymers. They are soft solids that changes irreversibly into insoluble polymer. They are not reusable. For example, bakelite.
Fig.11. Structure of bakelite
-
Thermoplastic Polymers are linear or slightly branched polymers which becomes soft on heating while becomes hard on cooling. They are moldable above a specific temperature. For example, polythene, polystyrene etc.
Fig.12. Structure of polystyrene
Uses of Polymers
-
They are used in industries for manufacturing Plastic bags, Bottles, Crates, Ropes, Water pipes and Insulation on electricity cables
-
Fillings for teeth
-
Dressings for cuts
-
Waterproof coatings for fabrics
-
Used in toys, cabinets, packaging etc.
Watch this Video for more reference
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free