Polymerization and Some Important Polymers
Polymerization is the process of formation of large molecules (polymers) by combination of large number of small molecules (monomers). It can also be defined as the process in which monomer molecules react together in a chemical reaction to form polymer chains or three-dimensional networks.
 Polymers are formed in two general ways.
Polymers are formed in two general ways.
-
In chain-reaction polymerization or addition polymers, there is a series of reactions each of which consumes a reactive particle and produces another, similar particle; each individual reaction thus depends upon the previous one. The reactive particles can be free radicals, cations, or anions. A typical example is the polymerization of ethylene. Here the chain-carrying particles are free radicals, each of which adds to a monomer molecule to form a new, bigger free radical.
Rad. + CH2 = CH2 → RadCH2CH2- RadCH2CH2CH2CH2- → etc.
RadCH2CH2CH2CH2- → etc.

-
In step reaction polymerization, there is a series of reactions each of which is essentially independent of the preceding one; a polymer is formed simply because the monomer happens to undergo reaction at more than one functional group. A diol, for example, reacts with a dicarboxylic acid to form an ester; but each moiety of the simple ester still contains a group that can react to generate another ester linkage and hence a larger molecule, which itself can react further, and so on.

-
Free-radical vinyl polymerization: In we discussed briefly the polymerization of ethylene and substituted ethylenes under conditions where free radicals are generated — typically in the presence of small amounts of an initiator, such as a peroxide. Reaction occurs.

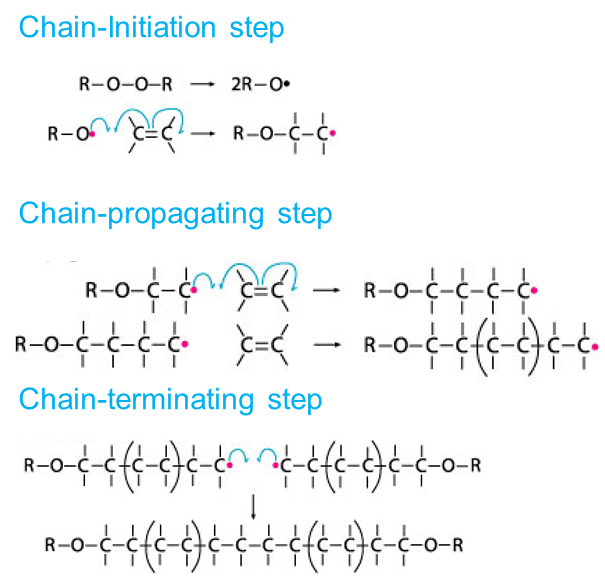
-
At the doubly bonded carbons — the vinyl groups — and is called vinyl polymerization. A wide variety of unsaturated monomers may be used, to yield polymers with different pendant groups (G) attached to the polymer backbone. For example.

-
Ziegler-Natta Polymerisation:
Addition polymerisaion which takes place in the presence of zigeler-Natta catalyst.
Gives linear, stereo-regular polymers.
High density polyethene is formed by this process. -
Copolymerization: So far, we have discussed only polymerisation of a single monomeric compound to form a homopolymer, a polymer made up — except, of course, at the two ends of the long molecule — of identical units.Now, if a mixture of two (or more) monomers is allowed to undergo polymerization, there is obtained a copolymer a polymer that contains two (or more) kinds of monomeric units in the same molecule.
Some Important Polymers
Polythene
Low-Density Polythene
-
Obtained by the polymerisation of ethene under high pressure of 1000 to 2000 atmospheres, and at a temperature of 350 K to 570 K, in the presence of traces of dioxygen or a peroxide initiator (catalyst)
-
Chemically inert, and tough but flexible
-
Poor conductor of electricity
High-Density Polythene
-
Formed by the addition polymerisation of ethene in a hydrocarbon solvent at a temperature of 333 K to 343 K and under a pressure of 6-7 atmospheres
-
Catalyst used − Triethylaluminium and titanium tetrachloride (Ziegler-Natta catalyst)
-
High density is due to close-packing
-
Chemically inert, and more tougher and harder than low density polythene
-
Used for manufacturing buckets, dustbins, bottles, etc.
Polytetrafluoroethene (Teflon)
- Catalyst used in preparation − Free radical or per-sulfate
- Chemically inert and resistant to attack by corrosive reagents
- Used for making oil seals and gaskets, and for non-stick-surface-coated utensils
Polyacrylonitrile
-
¨Used as a substitute for wool in making commercial fibres as orlon or acrilan
Natural Rubber:
Natural rubber is an addition polymer of isoprene (2-methyl-1,3-butadiene). Rubber has an average chain length of 5000 monomer units of isoprene.
The rubber in which the arrangement of carbon chain is trans with respect to the double bond is known as Gutta Percha and this is the natural rubber obtained from bark of various trees.
Natural rubber is sticky material. This disadvantage is removed by 'VULCANISATION' which involves addition of sulphur to rubber and heating the mixture. sulphur forms short chains of sulphur atoms that link two hydrocarbon (isoprene) units together.

When tension is applied the chains can strengthen out but they cannot slip over each other because of sulphur bridges. Thus rubber can be stretched only to a certain extent and hydrocarbon chains have the tendency to regain their shape when tension is removed. Vulcanised rubber is thus stronger and less sticky than the natural rubber.
Synthetic Rubber:
(Polychloroprene) or Neoprene) It is obtained by free radical polymerisation of chloroprene in

It is a thermoplastic and need not to be vulcanised. It is a good general purpose rubber and superior to natural rubber as it is resistant to the reaction of air, heat, light chemicals, alkalis and acids below 50% strength. It is used for making transmission belts, printing rolls and flexible tubing employed for conveyence of oil and petrol.
Buna Rubbers:
Butadiene polymerises in the presence of sodium to give a rubber substitute viz. BuNa. It is of two types
-
Buna - N or GRA: it is synthetic rubber obtained by copolymerisation of one part of acryl nitrile and two parts of butadiene.
.jpg)
It is more rigid responds less to heat and very resistant to swelling action of petorol, oils and other organic solvents. -
Buna -S or GRS (General purpose Styrene rubber): It is a copolymer of three moles of butadiene and one mole of styrene and is an elastomer. It is obtained as a result of free radical copolymerisation of its monomers
.
-
It is generally compounded with carbon black and vulcanised with sulphur. It is extremely resistant to wear and tear and finds use in manufacture of tyres and other mechanical rubber goods.
-
Teflon: It is polymer of tetrafluorethylene (F2C=CF2) which on polymerization gives Telfon.
nCF2=CF2 (—CF2—CF2—)n
(—CF2—CF2—)n
It is thermoplastic polymer with a high softening point (600K). It is very tough and difficult to work. It is inert to most chemicals except fluorine and molten alkali metals. It withstands high temperatures. Its electrical properties make it an ideal insulating material for high frequency installation. -
Nylon-66: It is a polymer resin. It is a condensation polymer formed by reaction between adipic acid and hexamethylene diamine. Both monomer units consist of 6 carbon atoms and therefore named nylon -66.


It is thermoplastic polymer when extruded above its melting point (536 K) through spinneret, it gives nylon fiber which is extremely tough and resistant to friction. It possess greater tensile strength, elasticity and lusture than any natural fiber. It is chemically inert and is fabricated into sheet, bristles and textile fibres. -
Nylon 6 or Perolon - L: A polyamide is prepared by prolonged heating of caprolactam at 530 - 540 K.

The fiber is practically identical to Nylon in properties
Polyesters
Polycondensation products of dicarboxylic acids and diols
Example: Dacron or terylene − manufactured by heating a mixture of ethylene glycol and terephthalic acid at 420 to 460 K.
Catalyst used: Zinc acetate-antimony trioxide
Dacron fibre is −
-
crease resistant
-
used in blending with cotton and wool fibers
-
as glass-reinforcing materials in safety helmets
Phenol-Formaldehyde Polymer

Novolac, obtained on heating with formaldehyde, undergoes cross- linking to form an infusible solid mass called bakelite.

Used for making combs, phonograph records,electrical switches and handles of various utensils.
Plasticiser
-
Plasticiser is an organic compound that dissolves in the polymer and allows the polymer chains to slode past one another. This makes polymer more flexible.
-
Dibutylphthalate is a commonly used plasticiser.

Melamine-Formaldehyde Polymer

Used in the manufacture of unbreakable crockery
Biodegradable & Nonbiodegradable Polymers
Biodegradable polymers are those polymers which degrade on their own by the action of microorganisms. All the natural polymers are biodegradable.
Example . PHBV Polymer (Polyhydroxy butyrate-co- β-hydroxy valerate), Dextron, Nylon-2-nylon-6
The non-biodegradable polymers do not undergo the environmental degradation processes and get accumulated as harmful solid waste materials.Synthetic polymers are non biodegradable.
PHBV Polymer
Polymer (Polyhydroxy butyrate-co- β-hydroxy valerate)
Monomers are 3-hydroxybutanoic acid and 3-hydroxypentanoic acid.

Dextron
Monomers are glycolic acid and lactic acid.

Nylon-2-nylon-6
Monomers are glycine and aminocaproic acid.


Q1.An example of biopolymer is
(a) teflon
(b) neoprene
(c) nylon-66
(d) DNA
Q2.In elastomer, intermolecular forces are
(a) strong
(b) weak
(c) zero
(d) None of these
Q3. Natural rubber is a polymer of
(a) butadiene
(b) isoprene
(c) 2-methylbutadiene
(d) hexa-l,3-diene
Q4. 
(a) addition polymer’
(b) thermosetting polymer
(c) homopolymer
(d) copolymer
Q5.Which of the following is an example of thermosetting polymer?
(a) Polythene
(b) PVC
(c) Neoprene
(d) Bakelite
Q6. A condensation polymer among the following is
(a) dacron
(b) PVC
(c) polystyrene
(d) teflon
Q7. On the basis of mode of formation, polymers can be classified
(a) as addition polymers only
(b) as condensation polymers only
(c) as copolymers
(d) as addition and condensation polymers
Q8. Ebonite is
(a) natural rubber
(b) synthetic rubber
(c) highly vulcanized rubber
(d) polypropene
Q9. Which is not a macromolecule?
(a) DNA
(b) Starch
(c) Palmitate
(d) Insulin
Q10. Which of the following is not an example of addition polymer?
(a) Polystyrene
(b) Nylon
(c) PVC
d) Polypropylene

|
Q.1 |
Q.2 |
Q.3 |
Q.4 |
Q.5 |
Q.6 |
Q.7 |
Q.8 |
Q.9 |
Q.10 |
|
d |
b |
b |
d |
d |
a |
d |
c |
c |
b |
Related Resources
Click here to go through the past year papers of IIT JEE
You can also refer to the syllabus of IIT JEE
To read more, Buy study materials of Polymers comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free