Nomenclature of Alcohols, Phenols and Ethers
Table of Content |
Nomenclature of Alcohols: Alcohol can be divided into three classes, which are as follows:
-
Monohydric Alcohol
-
Dihydric Alcohol
-
Trihydric Alcohol
There nomenclature is as following:
-
Monohydric Alcohol: Monohydric alcohols have general formula CnH2n+1OH where n = 1, 2, etc. or it can also be written as R-OH where R describes any alkyl group. There are three systems for the nomenclature of Monohydric Alcohol.
-
Common System: In this system, monohydric alcohols are called Alkyl Alcohol. Their names are derived by adding the name alcohol after the name of the alkyl group present in the molecule.
Example: The compound CH3-OH is a result of the addition of one methyl group with an alcohol group. Hence it name should be Methyl Alcohol.
CH3-OH a Methyl + Alcohol = Methyl Alcohol
Fig. Formation of Isopropyl Alcohol
-
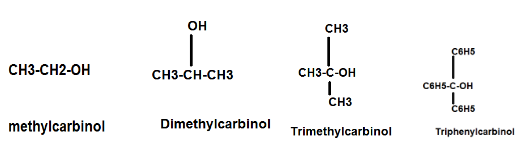
Carbinol System: In this system methyl alcohol (CH3OH) is called Carbinol while other alcohols are named as alkyl or aryl derivatives of carbinol.
Example: CH3-CH2-OH is termed as methylcarbinol and CH3-CH2-CH2-OH is called Ethylcarbinol. Some other examples are as follows:
Fig. Examples of Nomenclature of some Monohydric Alcohol in carbinol system
-
IUPAC System: In IUPAC nomenclature, alcohols are termed as Alkanols. The name of any alcohol can be derived by replacing the last ‘e’ from the name of the corresponding alkane by the suffix ‘–ol’. Then the longest carbon chain containing the OH group is selected as the parent chain and numbered the longest chain in such a way that the carbon atom carrying the OH group gets the smaller number. The position of the substituents is then shown by suitable numbers allotted to their respective carbon atom.
Example: The compound CH3-OH in common name called as Methyl Alcohol but in the IUPAC it is termed as Methanol. It should be noted that the last ‘e’ of the methane is replaced by ‘ol’ which indicates the presence of an alcohol group. The IUPAC name of few monohydric alcohols is given below:
In the naming of the Cyclic monohydric alcohols prefix ‘cyclo’ is used in writing the common or the IUPAC names of the straight chain alcohols. In the common system, the position of the substituents, are indicated by Greek alphabets: α, β, ϒ …etc. and in the IUPAC system numerical prefixes 1,2,3,4…..is used, with the carbon atom carrying the –OH group being numbered by 1.
Example of Cyclic Alcohol
-
Dihydric Alcohols: Dihydric alcohol have general formula (CH2)n(OH)2, where n= 2,3,4…. Etc. Because of their sweet taste, these are commonly known as Glycols. Depending upon the relative position of the two hydroxyl group, they are classified as α, β, ϒ…..ω-glycols, etc. Thus α glycol is 1,2-glycol, β glycol is 1,3-glycol and in Ψ-glycol the OH group are attached to the terminal carbon atom. These are named by the following two systems:
- Common System: In common system, α- glycols are named by adding the word Glycol after the name of the alkene.
Example
Fig. Example of dihydric alcohol in common system
In contrast β, ϒ … ω – glycols are named as the corresponding polymethylene glycols.
Example: HO-CH2CH2CH2-OH HO-CH2CH2CH2CH2CH2-OH
Trimethylene glycol ( A β-glycol) pentamethylene glycol
-
IUPAC system: In this system, glycols are called as Diols and their class name is Alkanediols. The two hydroxyl group position is indicated by Arabic numerals.
Example
Fig. Examples of dihydric alcohol in IUPAC system
-
Trihydric Alcohol: The general formula of trihydric alcohols is (CH2)n(OH)3 where n = 3, 4, 5 …etc. In this system there is no general rule for naming these alcohols. So there is only one IUPAC rule which is described as below:
-
IUPAC System: In this system trihydric alcohol, are called Alakanetriols. The position of the OH group is indicated by Arabic Numerals.
Fig. Examples of trihydric alcohol in IUPAC system
Nomenclature of Phenols
Phenols can also be divided into three classes which are monohydric phenols, dihydric phenols and trihydric phenols. There nomenclature is as following:
-
Monohydric Phenols: The simplest derivative of benzene is called Phenol. It is common name as well as an accepted IUPAC name. Both in the common and in the IUPAC system, substituted phenols are named as the derivatives of phenols. In the common system , the substituent position present on the benzene ring with respect to –OH group is indicated by adding the prefix such as ortho (o-) for 1:2, meta (m-) for 1,3 and para (p-) for 1,4. In spite of this, some phenols are still best known by their Trivial Names.
However, in the IUPAC system, the position of the substituent w.r.t –OH group is indicated by Arabic numeral, with the carbon carrying the OH group being numbered 1.
The common and the IUPAC names of such phenols are given below:
Fig. Examples of monohydric phenol
Phenol containing a carbonyl group such as aldehyde, ketonic, carboxyl or an ester group is named as hydroxyl derivatives of the parent aromatic compound.
Example
Fig. Monohydric phenols
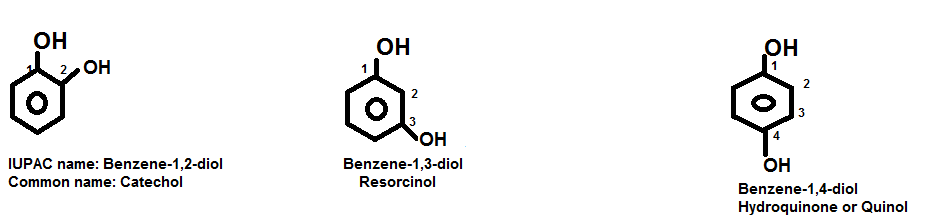
- Dihydric and Trihydric Phenols: In the IUPAC system, di-. Tri- and polyhydric phenols are named as Hydroxyl Derivatives of Benzene. However, they are commonly known by their Trivial Names. For example:
Fig. Examples of dihydric and trihydric phenols
Nomenclature of Ethers
-
Common System: The common name of ethers is derived by naming the two alkyl or aryl group linked to the oxygen atom as separate words in alphabetical order and adding the word ether. In case of symmetrical ethers, the prefix di is used before the name of the alkyl or the aryl group.
-
IUPAC system: In the IUPAC system, ethers are named as Alkoxyalkanes. The ethereal oxygen is taken with the smaller alkyl group and forms a part of the alkoxy group while the larger alkyl group is considered to be the part of the alkane.
Fig. Formula, common name and IUPAC name of some ethers
Watch this Video for more reference
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free