Chemical Properties of Aldehydes & Ketones
Table of Content |
|
|
-
Oxidation? Aldehydes & Ketones

Tollen’s Reagent
A specific oxidant for RCHO is Ag(NH3)2+

Tollen’s test chiefly used for the detection of aldehydes.
Tollen’s reagent does not attack carbon-carbon double bonds.
Strong Oxidants: Ketones resist mild oxidation, but with strong oxidants at high temperature they undergo cleavage of C – C bonds on either sides of the carbonyl gorup.

CH3COR are readily oxidised by NaOI (NaOH + I2) to iodoform, CHI3, and RCO2Na
Example:

-
Reduction Aldehydes & Ketones
a) Reduction to alcohols

Aldehydes → 1° alcohols; Ketones → 2°alcohols
Example:


-
Addition Reactions of Carbonyl Compounds
The C of the carbonyl group is electrophilic

and initially forms a bond with the nucleophile

a) Addition of cyanide
b) Addition of bisulfite

Example


c) Addition of derivative of ammonia

|
|
H2N – G |
Product |
|
|
H2N – NH2 |
Hydrazine |
> C = N – NH2 |
Hydrazone |
|
H2N – NH – C6H5 |
Phenylhydrazine |
> C = N – NHC6H5 |
Phenylhydrazone |
|
H2N – NH – C – NH2 || O |
Semicarbazide |
> C = N –NHCONH2 |
Semicarbazone |
|
|
2, 4-Dinitrophenyl hydrazine |
|
2, 4 dinitrophenylhydrazone (bright orange or yellow precipitate used for identifying aldehydes and ketones |
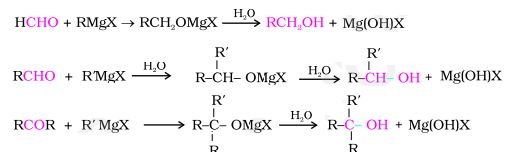
d) Addition of Grignard Reagent

e) Addition of hydroxylamine

f) Addition of Alcohols; Acetal Formation

In H3O+, RCHO is regenerated because acetals undergo acid catalyzed cleavage much more easily than do ethers. Since acetals are stable in neutral or basic media, they are used to protect the – CH = O group.
-
Halogenation of Ketones
?a.


-
Acidity of a-Hydrogens
Carbonyl group largely determines the chemistry of aldehydes and ketones.
But how the carbonyl group strengthens the acidity of hydrogen atoms attached to the a-carbon and by doing this gives rise to a whole set of chemical reaction?

-
Aldol Condensation
Substrate  aldehyde or ketone containing a hydrogen
aldehyde or ketone containing a hydrogen
Reagent  Dilute Base
Dilute Base
Product  ß-hydroxyaldehyde or b-hydroxyketone
ß-hydroxyaldehyde or b-hydroxyketone
Under the influence of dilute base or dilute acid, two molecules of an aldehyde or ketone may combine to form a b-hydroxy aldehyde or ketone.
The a-carbanion generated by the base from one molecule of ald. Or ketone adds to the carbonyl carbon of the other molecule and the two molecules condense and form b-hydroxy aldehyde or ketone. Let us consider the mechanism for the OH catalysed aldol condensation of acetaldehyde:

Aldol condensations are reversible, and with ketones the equilibrium is unfavourable for condensation product. b-hydroxycarbonyl compounds are readily dehydrated to give a-b-unsaturated carbonyl compounds. With Ar on b-carbon, only dehydrated product is isolated.
-
Crossed Aldol Condensation
An aldol condensation between two different carbonyl compounds so called crossed aldol condensation – is not always useful as a mixture of four different possible products may be obtained.
Under certain condition, a good yield of a single product can be obtained from a crossed aldol condensation. One reactant contains no a-hydrogens and therefore is incapable of condensing with itself (eg. Aromatic aldehydes or formaldehyde).

-
Cannizzaro Reaction
Discussion: In the presence of concentrated alkali, aldehydes containing no - a-hydrogens undergo self-oxidation and reduction to yield a mixture of an alcohol and a salt of a carboxylic acid. This reaction is known as Cannizzaro – reaction.
Two successive additions are involved.
a) Addition by hydroxide ion in first step
b) Addition of hydride ion in the next step

This explains the Crossed Cannizzario reaction involving formaldehyde to take place in the way that it does.
ArCHO + HCHO → HCO2Na+ + ArCH2OH
On both electronic and steric grounds, the step 1 is faster for HCHO. Hence becomes the hydride donor in the next step.

-
Perkin Condensation?

Ester can be condensed with aromatic aldehydes in the presence of alkoxides; thus benzaldehyde and ethylacetate in the presence of sodium ethoxide, give ethyl cinnamate,
C6H5CH = CHCOOC2H5.

-
Halogenation of Ketones?

Examples

The Haloform Test depends upon the fact that the three hydrogens on the same carbon atom are successively replaced by halogen. Taking acetone as an example we see that the carbon that suffers the initial substitution to the preferred site undergoes further substitution.

Electron withdrawal by halogen makes hydrogens on the carbon to which halogen has already being attached more acidic and hence more readily removed by base to give further substitution.
Electron withdrawal by three halogens makes –CX3 comparatively weakly basic (for a carbanion) and hence acts as a good leaving group.
Thus both essential aspects of the haloform reaction –– regiospecificity of halogenation, and cleavage –– are controlled by the factor; stabilization of a carbanion through electron withdrawal.
-
Claisen Condensation,Formation of b-Keto Esters
An a–hydrogen in an ester, like an a-hydrogen in an aldehyde or ketone, is weakly acidic, because, the carbonyl group helps to accommodate the negative charge of the carbanion.
When ethyl acetate is treated with sodium ethoxide, and the resulting mixture is acidified ethyl 3–oxobutanoate, generally known as ethyl acetoacetate or acetoacetate or acetoacetic ester is obtained.

Ethyl accetoacetate is the ester of a b-Keto acid; its preparation illustrates the reaction known as the Claisen Condensation.

-
Crossed Claisen Condensation
Examples:
Ketones (but not aldehydes) undergo a crossed Claisen Condensation with ester.
Example:

-
Decarboxylation of b-Keto Acids
b-Keto esters are normally prepared by Claisen Condensation. Hydrolysis of the b-keto ester gives the b-keto acids which are very easy to decarboxylate simply by heating. Decarboxylation of free acetoacetic acid involves transfer of the acidic hydrogen to the keto-group followed by loss of carbon-dioxide via a cyclic 6-membered T.S.

-
Addition of Ammonia
Aldehydes react with ammonia to form aldehyde ammonia

The aldehyde ammonia is unstable and lose water immediately to form aldimine. The dehydration product is not usually obtained because, in most cases, it immediately polymerises to form cyclic trimers.

When treated with ammonia, formaldehyde does not form an aldehyde – ammonia, but gives instead hexamethylenetetramine, used in medicine as a urinary antiseptic under the name Urotoropine.

Ketones also give ketone-ammonia but these cannot be isolated. Acetone reacts slowly with ammonia to form acetone ammonia and then a complex compound.

Acetone upon treatment with ammonia at higher temperature give acetoneammonia.

Aldimines, Schiff’s bases or azomethines are formed when aldehydes react with aliphatic primary amines, which is removed by slow distillation.
-
Meerwein – Ponndorf – Verley Reduction
The carbonyl compound is heated with aluminium isopropoxide in isopropanol solution, the isopropoxide is oxidised to acetone, which is removed by slow distillation.

The reducing agent is specific for the carbonyl group, and so may be used for reducing aldehydes and ketones containing some other functional group that is reducible e.g., a double bond or a nitro group.

-
Pinacol-Pinacolone Rearrangement
(1, 2-methyl shift) upon treatment with hot dil. H2SO4, pinacol undergoes a rearrangement and dehydration to give a methyl ketone.

-
Tischenko Reaction
All aldehydes can be made to undergo the Cannizzaro reaction by treatment with aluminium ethoxide. Under these conditions the acids and alcohols are combined as the ester, and the reaction is then known as the Tischenko reaction; eg, acetaldehyde gives ethyl acetate, and propionaldehyde gives propyl propionate

-
Benzoin Condensation:
When refluxed with aqueous ethanolic potassium cyanide benzaldehyde forms benzoin.

-
Sommelet's Reaction
Benzaldehyde is produced when benzyl chloride is refluxed with hexamethylenetetramine in aqueous ethanolic solution followed by acidification and steam distillation.
C6H5CH2Cl + (CH2)6N4→ C6H5CHO
-
Analysis of Aldehydes And Ketones
Aldehydes and ketones are characterized through the addition to the carbonyl group of nucleophilic reagents, especially derivatives of ammonia. All aldehyde or ketone will, for example react with 2,4-dinitrophenylhydrazine to form an insoluble yellow or red solid.
Aldehydes are characterized, and in particular are differentiated from ketones through their ease of oxidation: aldehydes give a positive test with Tollen's reagent; ketones do not.
Aldehydes are also, of course, oxidized by many other oxidizing agents : by cold, dilute, neutral KMnO4 and by CrO3 in H2SO4.
A highly sensitive test for aldehydes is the Schiff’s test.
Aldehydes and ketones are generally identified through the melting points of derivatives like 2,4-dinitrophenylhydrazones, oximes, and semicarbazones.
Methyl Ketones are characterized through the iodoform test.
Aldehydes can be oxidised by Fehling's solution.
Fehling's solution, an alkaline solution of cupric ion complexed with tartarate ion (or Benedict's solution, in which complexing is with citrate ion); the deep-blue colour of the solution is discharged, and red cuprous oxide precipitates.
Fehling's solution is made by mixing, Fehling A solution, which contains copper sulphate,
+ Fehling B solution, which contains sodium hydroxide and Rochelle salt (Sodium Potassium Tartarate). During the oxidation of aldehydes to acids, the cupric ions are reduced to cuprous ions which are precipitated as red cuprous oxide.
RCHO + 2Cu2+ + 3-OH → R-CO-2 + 2Cu+ + 2H2O
2Cu+ + 2-OH → Cu2O¯ + H2O
Cuprous oxide (red)
You can also refer to Organic Chemistry Revision Notes and IIT JEE Chemistry Syllabus





