Cleansing Agents
Table of Content |
Definition of Cleansing Agents
Chemical substances which concentrate on the surface of the solution to form surface films to reduce surface tension of the solution and to help in removing dirt and dust by emulsifying grease are called Cleansing Agents or Surface active agents or Surfactants.
The molecules of a cleansing agent contain two characteristics group; one of which is Water Soluble (also called Hydrophilic) and the other is Oil Soluble (also called Lyophilic or Lipophilic). Surfactants are of two types:
-
Soaps
-
Synthetic Detergents.
Detailed description is given below:
1. Soaps
Soaps are potassium or sodium salt of higher fatty acid such as lauric acid (C11H23COOH), palmitic acid (C15H31COOH), stearic acid (C17H35COOH), oleic acid (C17H35COOH) or linoleic acid (C17H31COOH). Soap is formed by heating fat or oil with aqueous sodium hydroxide (NaOH) solution. This reaction is called Saponification.
Fig. Saponification Reaction (formation of soap)
Only sodium and potassium soaps are soluble in water and are used for cleaning purposes. Potassium soaps are soft to skin as compared to sodium soaps. Potassium soaps are prepared by hydrolysis of oils and fat with aqueous potassium hydroxide instead of sodium hydroxide.
Types of Soaps
All soaps are prepared by boiling fats and oils with a suitable soluble hydroxide. However different raw materials can be used to make different soaps. Some of them are as follows:
-
Toilet Soaps: It is prepared by using better quality grades of fats and oils. Colour and perfumes are also added to make them attractive.
-
Floating Soaps: It is prepared by beating air bubbles into the product before hardening.
-
Medicated Soaps: They are prepared by adding some antiseptics like savlon, dettol etc. In some medicated soaps deodorants are also used for good smell.
-
Shaving Soaps: They contain glycerol to prevent rapid drying. While preparing a gum called Rosin is added to them as it forms sodium rosinate which lathers well.
-
Laundry Soaps: They contain fillers like sodium silicate, borax and sodium carbonate.
-
Soap Chips: They are made by running a thin sheet of melted soap on to a cool cylinder and scraping off the soaps in small broken pieces.
Advantages of Using Soap as a Cleansing Agents
Soap is a good cleansing agent and 100% biodegradable and hence micro-organism present in sewage water can completely oxidise soap to CO2 and hence it does not create any pollution problems.
Disadvantages of Soaps
Soaps have following two disadvantages:
-
Soaps cannot be used in hard water since magnesium and calcium ions present in hard water produce curdy white precipitates of calcium and magnesium salts of fatty acid. For Example:
2 C17H35COONa + CaCl2 → (C17H35COO)2Ca + 2 Nacl
soluble sodium stearate calcium chloride Insoluble calcium stearate (scum)
-
Soaps cannot be used in acidic solution since acid precipitate the insoluble free fatty acid which adhere to the fabric and thus reduce the ability of soaps to remove oils and grease from fabrics.
2. Detergents
Detergents are defined as Ammonium, Sulphonate or Sulphate slats of long chain hydrocarbons containing 12-18 carbon atoms. Commonly, detergents are the sodium salts of long chain sulphonic acid.
Detergents can also be used in hard water. Because calcium or magnesium salts of detergents like their sodium salts are also soluble in water. Hence detergents do not forms curdy white precipitates with hard water and they can also be used in Acidic Solutions because fatty acids, sulphonic acid are soluble in water.
Classification, Synthesis and uses of Detergents
Detergents are of three types:
-
Anionic Detergents
-
Cationic Detergents
-
Non-ionic Detergents
Detailed descriptions are as follows:
-
Anionic Detergents: They are called Anionic Detergents because a large part of their molecules are anions and it is the anionic part of the molecule which is involved in their cleansing action. These are of two types:
-
Sodium Alkyl Sulphates: They are obtained from the long straight chain alcohols containing 12-18 carbon atoms treated with conc. H2SO4 followed by neutralization with NaOH. Some important examples are sodium laurylsulphate (C11H23CH2OSO3-Na+) and sodium stearyl sulphate (C17H35CH2OSO-3Na+).
C11H23CH2OH --- → C11H23CH2OSO3H – → C11H23CH2OSO-3Na+
lauryl alcohol concH2SO4 lauryl hydrogen sulphate NaOH sodium lauryl sulphate
-
Sodium Alkylbenzenesulphonates: These detergents are the sodium salt of long chain alkylbenzenesulphonic acid. These are obtained by Friedel-Crafts alkylation of benzene with a long chain alkyl halide or an alkene or an alcohol followed by sulphonation and neutralization with NaOH. Examples are sodium 4-(2-dodecyl)benzenesulphonate (SDS) and sodium 4-(2-dodecyl)benzenesulphonate.
Fig. Structure of sodium 4-(2-dodecyl)benzenesulphonate.
Alkylbenzenesulphonates in which the phenyl group is either attached to the end of the long alkyl chain or any other secondary position, have high biodegradability and hence are called Soft Detergents while alkylbenzenesulphonates have highly branched alkyl group and have poor biodegradiblity and hence are called Hard Detergents.
To differentiate these detergents on the basis of biodegradability, alkylbenzenesulphonates having long straight chains such as sodium 4-(2-dodecyl)benzenesulphonate are called LAS Detergents while those having branched chain are called ABS Detergents.
-
Cationic Detergents: They are called Cationic Detergent because major parts of their molecule are cation and it is the cationic part of the molecules which is involved in their cleansing action. They are called as Invert Soaps because their cleansing action is due to the presence of cations rather than anions. They are quaternary ammonium salts containing long chains of one or more alkyl groups. It is used as a germicide.
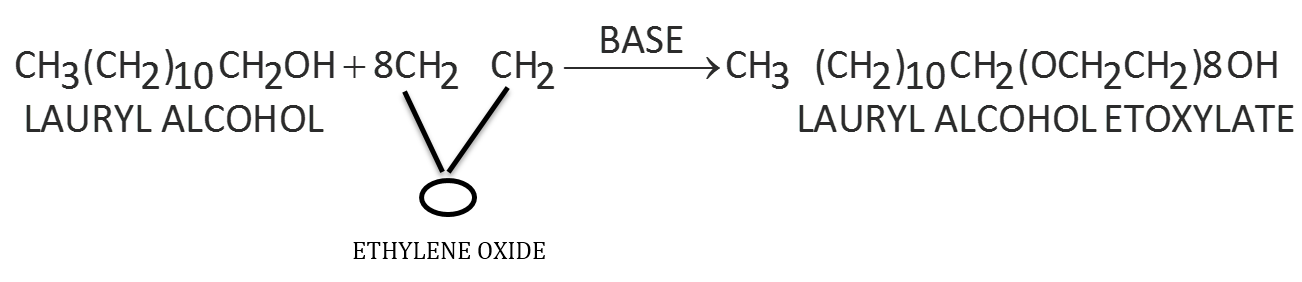
- Non-ionic Detergents: These detergents do not contain any ion. These are actually esters of high molecular mass alcohol obtained by reaction between polyethylene glycol and stearic acid.
These may also be obtained by long chain alcohol treatment with excess of ethylene oxide in presence of a base.
Advantages of Synthetic Detergents
Their advantages are as follows:
-
Synthetic detergents can be used in hard water without any wastage while some of the soaps gets wasted in hard water.
-
Synthetic detergents can be used in acidic medium
-
Synthetic detergents are more soluble in water and hence produce lather moe easily. Some synthetic detergents produce lather in cold water also.
-
Synthetic detergents decrease the surface tension of water to a greater extent and hence have a stronger cleansing action than soap.
Disadvantages of Detergents
Detergents have highly branched hydrocarbon chain which causes pollution in rivers and other waterways. The reason being that, the side chain of the detergent stop bacteria from attacking and breaking the chains. This results in the slow degradation of detergent molecule leading to their accumulation. Effluents containing such detergents reach the river, ponds etc. these persist in water even after sewage treatment and cause foaming in rivers, streams and ponds, and thus water gets polluted.
Since un-branched chains are more vulnerable to attack by bacteria, therefore, in most of the detergents used these days, the branching is kept to a minimum so that the detergents become easily biodegradable to prevent pollution.
Frequntly Asked Questions (FAQs)
Q1. What is a degreaser?
Sol. The chemical substance that removes the grease or oils from the surface of floor, machines by dissolving in it are called Degreaser.
Q2. Is bleach an acid or an alkali?
Sol. Bleach is an alkali (chemical substance) which is used to remove colour with the help of oxidation.
Watch this Video for more reference