Air Pollution
Table of Content |
 Air pollution is defined as the addition of undesirable material into the atmosphere either due to natural phenomena or due to human activities. Air pollution is the atmosphere condition in which the presence of certain contaminants produces harmful effects on man and his environment. These substances include:
Air pollution is defined as the addition of undesirable material into the atmosphere either due to natural phenomena or due to human activities. Air pollution is the atmosphere condition in which the presence of certain contaminants produces harmful effects on man and his environment. These substances include:
(i) Gases such as oxides of sulphur, CO, oxide of N2 ,hydrocarbons, ozone and other oxidents
(ii) Particulate matter such as dust, smoke, fumes etc.
(iii) Radioactive material & many others.
The chemical substances causing air pollution are known as air pollutants.
More than 90% of the air pollution is caused by oxides of S,
oxides of C, oxides of N, volatile organic compounds and particulate matter.
-
Major Sources of Air Pollution
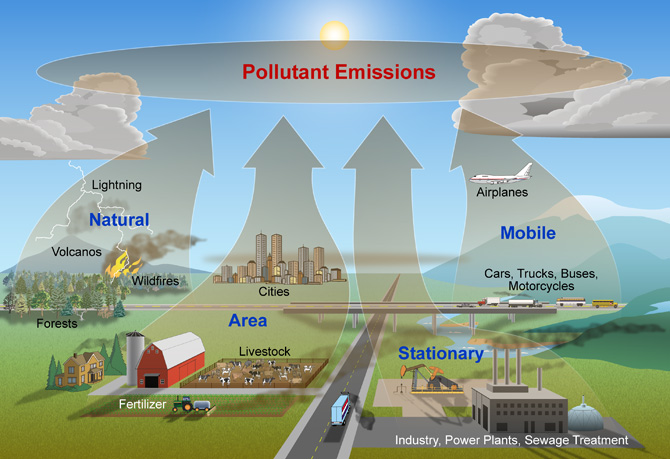
The air pollution is caused by both human activities and natural phenomena.
The natural sources of air pollution include volcanic eruptions, forest fires, pollen grain dispersal, evaporation of volatile organic compounds, microbial decomposition of organic matter, wind erosion of soil and nuclear radioactivity.
Human activity like burning of fuels for energy is the major source for air pollution. Some other human activities responsible for air pollution are.
-
Combustion of gasoline in the automobile
-
Deforestation: This increases the amount of carbon dioxide gas in air and the amount of oxygen in air gets decreased.
-
Fast Industrialization: The smoke of which is produced by industries contains gases like CO, CO2,H2S, NO & NO2, These industries are responsible for 20% of total air pollution.
-
Agricultural activities: The pesticides are added in the soil in order to kill the harmful pests. While doing so, a large amount of pesticides escape into environment by mixing with air.
-
Wars: The nuclear weapons are used in war which emits the harmful radiations.
-
Particulate Pollutants
-
Soot: produced by incomplete combustion of carbonaceous fossils fuels such as coal, fuel oil, natural gas, wood etc in insufficient supply of oxygen.
-
Metal particles: These are released by various metal finishing operation. The micro particles of toxic metal & SO2 gas present in the polluted atmosphere get absorbed on the particles rendering them highly toxic.
-
Metal oxides: They are generated by combustion of fuels containing metallic compounds.
-
Lead salts: Their source is lead tetraethyl (Pb(C2H5)4) which is added to gasoline to improve its antiknock property. In order to avoid deposition of PbO suitable amounts of C2H4Cl2 & C2H4Br2 are added to gasoline along with Pb(C2H5)4.
-
Fly ash: It originates from the combustion of high ash fossil. It contains partially burnt particles of the fuels.
-
Asbestos dust: It originates from industrial units manufacturing asbestos sheets, gaskets ropes etc. Asbestos flowing & asbestos insulations also contribute towards asbestos dust in the atmosphere.
-
Solid Hydrocarbons: These are emitted from petroleum refineries & comprise of paraffins, olefins & aromatics.
-
Dust Particulates: Originate from natural, domestic, industrial or agricultural sources. These are thrown into atmosphere by volcanic eruptions, blowing of dust by wind, mining operations etc.
-
Acid mist: Sulphuric acid mist is produced when SO3 present in the atmosphere comes in contact with moisture. Nitric acid mist is produced when oxides of nitrogen, viz, NO & NO2, undergo the series of reactions in the atmosphere.
Harmful effects of particulates
-
Effect on human beings: Affect the human respiratory system & cause several respiratory illnesses.The particles with small size are more harmful in this context. The particulates in fact, become the carriers of the toxic substances from the atmosphere to the human & cause big health hazards.
-
Effect on visibility: Particulates in the atmosphere cause scattering & absorption of sunlight & reduce the visibility.
-
Effect on Materials : The adverse effect of particulates on materials include corrosion of metals (when the atmosphere is humid), erosion & soiling of building, sculptures & painted surfaces & soiling of clothes & draperies.
-
Gaseous Pollutants
Related Links
Refer the below mentioned links to get an immediate solution to all queries on Organic chemistry:
To read more, Buy study materials of Environmental Chemistry comprising study notes, revision notes, video lectures, previous year solved questions etc. Also browse for more study materials on Chemistry here.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free
