Classification of Organic Comounds
Table of Content |
Classification of Organic Compounds Based on Structure
 ?
?
Refer to the following video for Classification of Organic Compounds:
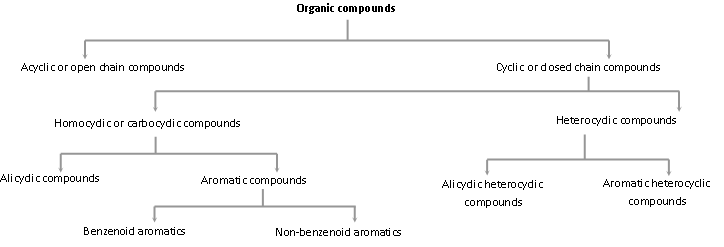
(1) Acyclic or open-chain compounds: Organic compounds in which all the carbon atoms are linked to one another to form open chains (straight or branched) are called acyclic or open chain compounds. These may be either saturated or unsaturated. For example,
![]()
![]()
![]()

These compounds are also called as aliphatic compounds.
Cyclic or closed-chain compounds: Cyclic compounds contain at least one ring or closed chain of atoms. The compounds with only one ring of atoms in the molecule are known as monocyclic but those with more than one ring of atoms are termed as polycyclic. These are further divided into two subgroups.
- Homocyclic or carbocyclic : These are the compounds having a ring or rings of carbon atoms only in the molecule. The carbocyclic or homocyclic compounds may again be divided into two types:
-
Alicyclic compounds: These are the compounds which contain rings of three or more carbon atoms. These resemble with aliphatic compounds than aromatic compounds in many respects. That is why these are named alicyclic, i.e., aliphatic cyclic. These are also termed as polymethylenes. Some of the examples are,
?
Aromatic compounds: These compounds consist of at least one benzene ring, i.e., a six-membered carbocyclic ring having alternate single and double bonds. Generally, these compounds have some fragrant odour and hence, named as aromatic (Greek word aroma meaning sweet smell).

These are also called benzenoid aromatics.
Non-benzenoid aromatics: There are aromatic compounds, which have structural units different from benzenoid type and are known as Non-benzenoid aromatics e.g. Tropolone, azulene etc.

(b) Heterocyclic compounds: Cyclic compounds containing one or more hetero atoms (e.g. O, N, S etc.) in the ring are called heterocyclic compounds. These are of two types:
Alicyclic heterocyclic compounds: Heterocyclic compounds which resemble aliphatic compounds in their properties are called Alicyclic heterocyclic compounds. For example,

Aromatic heterocyclic compounds: Heterocyclic compounds which resemble benzene and other aromatic compounds in most of their properties are called Aromatic heterocyclic compounds. For example,

Classification of Organic Compounds Based on Functional Groups
A functional group is an atom or group of atoms in a molecule that gives the molecule its characteristic chemical properties. Double and triple bonds are also considered as functional groups.
All compounds with the same functional group belong to the same class. Various classes of compounds having some of the common functional groups are listed in the table.
|
Class |
Functional group |
Class |
Functional group |
|
Olefins/Alkenes (ene) |
|
Acid halides (Alkanoyl halids) |
|
|
Acetylenes/Alkynes (yne) |
|
Amides (Alkanamides) |
|
|
Alkyl Halides |
|
Acid anhydrides (Alkanoic anhydrides) |
|
|
Alcohols (Alkanols) |
–OH (Hydroxy) |
Esters (Alkylalkanoates) |
|
|
Ethers (Alkoxyalkanes) |
|
Cyanides/Nitriles (Alkanenitrile) |
|
|
Aldehydes (Alkanals) |
|
Isocyanides |
– N C (Isocyano) |
|
|
|
Nitro compounds (Nitroalkanes)
|
(Nitro) |
|
(Alkanoic acid) |
|
Amines |
(Amino) |
Look here for Organic Chemistry Revision Notes, Chemistry Syllabus and Best books of Organic Chemistry.
View courses by askIITians


Design classes One-on-One in your own way with Top IITians/Medical Professionals
Click Here Know More

Complete Self Study Package designed by Industry Leading Experts
Click Here Know More

Live 1-1 coding classes to unleash the Creator in your Child
Click Here Know More

a Complete All-in-One Study package Fully Loaded inside a Tablet!
Click Here Know MoreAsk a Doubt
Get your questions answered by the expert for free