Purification of Organic Compounds
Table of Content |
A large number of methods are available for the purification of substances. The choice of method, however, depends upon the nature of substance (whether solid or liquid) and the type of impurities present in it. Following methods are commonly used for this purpose,
-
Simple crystallisation
-
Fractional crystallisation
-
Sublimation
-
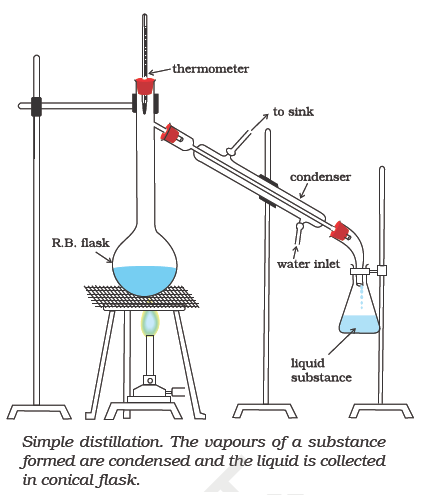
Simple distillation
-
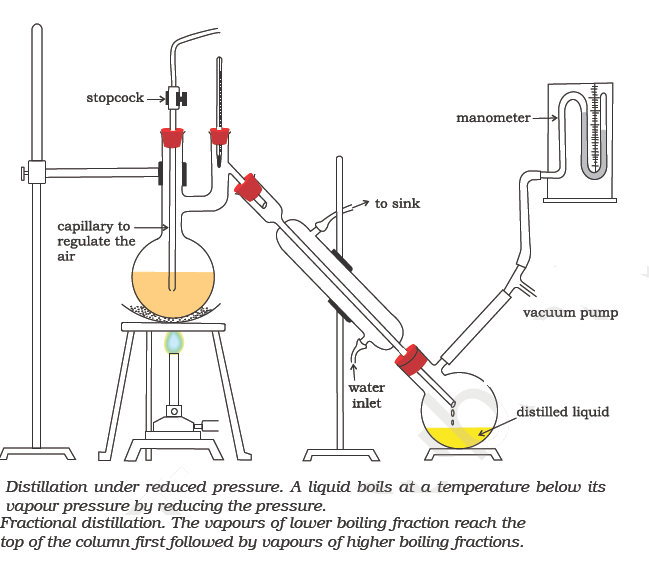
Fractional distillation
-
Distillation under reduced pressure
-
Azeotropic distillation
-
Chromatography
-
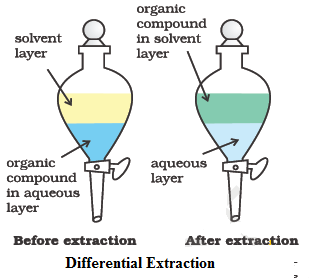
Differential extraction
-
Chemical methods
-
Simple Crystallisation
This is the most common method used to purify organic solids. It is based upon the fact that whenever a crystal is formed, it tends to leave out the impurities. For crystallisation, a suitable solvent is one (a) which dissolves more of the substance at higher temperature than at room temperature (b) in which impurities are either insoluble or dissolve to an extent that they remain in solution (in the mother liquor) upon crystallisation, (c) which is not highly inflammable and (d) which does not react chemically with the compound to be crystallized. The most commonly used solvents for crystallisation are: water, alcohol, ether, chloroform, carbon- tetrachloride, acetone, benzene, petroleum ether etc.
Examples :
- Sugar having an impurity of common salt can be crystallized from hot ethanol since sugar dissolves in hot ethanol but common salt does not.
- A mixture of benzoic acid and naphthalene can be separated from hot water in which benzoic acid dissolves but naphthalene does not.
-
Fractional Crystallisation
The process of separation of different components of a mixture by repeated crystallisations is called fractional crystallisation. The mixture is dissolved in a solvent in which the two components have different solubilities. When a hot saturated solution of this mixture is allowed to cool, the less soluble component crystallises out first while the more soluble substance remains in solution. The mother liquor left after crystallisation of the less soluble component is again concentrated and then allowed to cool when the crystals of the more soluble component are obtained. The two components thus separated are recrystallized from the same or different solvent to yield both the components of the mixture in pure form.
Fractional crystallisation can be used to separate a mixture of ![]() (less soluble) and KCl (more soluble).
(less soluble) and KCl (more soluble).
-
Sublimation
Certain organic solids on heating directly change from solid to vapour state without passing through a liquid state, such substances are called sublimable and this process is called sublimation.
The sublimation process is used for the separation of sublimable volatile compounds from non sublimable impurities. The process is generally used for the purification of camphor, naphthalene, anthracene, benzoic acid![]() , solid
, solid ![]() , Iodine and salicylic acid etc containing non-volatile impurities.
, Iodine and salicylic acid etc containing non-volatile impurities.
-
Simple Distillation
Distillation is the joint process of vapourisation and condensation. This method is used for the purification of liquids which boil without decomposition and contain non-volatile impurities. This method can also be used for separating liquids having sufficient difference in their boiling points. This method can be used to separate a mixture of
-
chloroform (b. p. 334 K) and aniline (b. p. 457 K)
-
ether (b. p. 308 K) and toluene (b. p. 384 K)
-
Fractional Distillation
 This process is used to separate a mixture of two or more miscible liquids which have boiling points close to each other. Since in this process, the distillate is collected in fractions under different temperatures, it is known as fractional distillation. This process is carried out by using fractionating columns. Fractionating column is a special type of long glass tube provided with obstructions to the passage of the vapour upwards and that of liquid downwards. This method may be used to separate a mixture of acetone (b. p. 330 K) and methyl alcohol (b. p. 338 K) or a mixture of benzene and toluene. One of the technological applications of fractional distillation is to separate different fractions of crude oil in petroleum industry into various useful fractions such as gasoline, kerosene oil, diesel oil, lubricating oil etc.
This process is used to separate a mixture of two or more miscible liquids which have boiling points close to each other. Since in this process, the distillate is collected in fractions under different temperatures, it is known as fractional distillation. This process is carried out by using fractionating columns. Fractionating column is a special type of long glass tube provided with obstructions to the passage of the vapour upwards and that of liquid downwards. This method may be used to separate a mixture of acetone (b. p. 330 K) and methyl alcohol (b. p. 338 K) or a mixture of benzene and toluene. One of the technological applications of fractional distillation is to separate different fractions of crude oil in petroleum industry into various useful fractions such as gasoline, kerosene oil, diesel oil, lubricating oil etc.
This method is used for the purification of high boiling liquids and liquids which decompose at or below their boiling points.
The crude liquid is heated in distillation flask fitted with a water condenser, receiver and vacuum pump. As the pressure is reduced, the liquid begins to boil at a much lower temperature than its normal boiling point. The vapour is condensed by water condenser and the pure liquid collects in the receiver.
Glycerol which decomposes at its boiling point (563 K) under atmospheric pressure can be distilled without decomposition at 453 K under 12 mm of Hg. Similarly, sugarcane juice is concentrated in sugar industry by evaporation under reduced pressure which saves a lot of fuel.
-
Steam Distillation
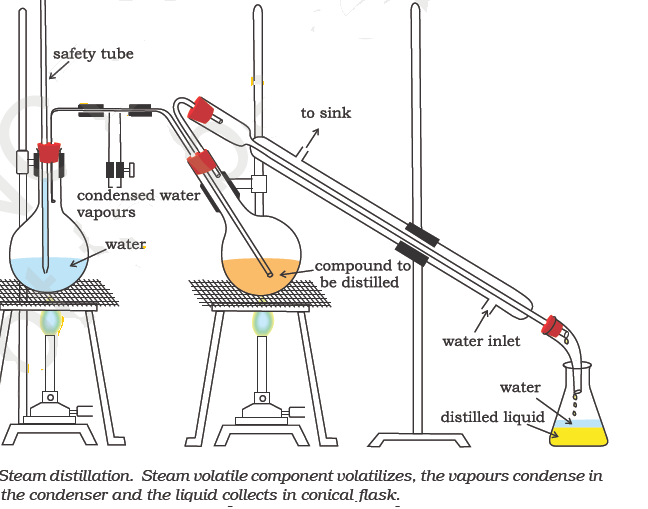 This method is applicable for the separation and purification of those organic compounds (solids or liquids) which (a) are insoluble in water (b) are volatile in steam (c) possess a high vapour pressure (10-15 mm Hg) at 373 K and (d) contain non-volatile impurities.
This method is applicable for the separation and purification of those organic compounds (solids or liquids) which (a) are insoluble in water (b) are volatile in steam (c) possess a high vapour pressure (10-15 mm Hg) at 373 K and (d) contain non-volatile impurities.
Aniline (b. p. 457 K) can be purified by steam distillation since it boils at a temperature of 371.5 K in presence of steam. Other compounds which can be purified by steam distillation are: nitrobenzene, bromobenzene, o-nitrophenol, salicylaldehyde, o-hydroxyacetophenone, essential oils, turpentine oil etc.
-
Azeotropic Distillation
Azeotropic mixture is a mixture having constant boiling point. The most familiar example is a mixture of ethanol and water in the ratio of 95.87: 4.13 (a ratio present in rectified spirit). It boils at 78.13oC. The constituents of an azeotropic mixture can't be separated by fractional distillation. Hence a special type of distillation (azeotropic distillation) is used for separating the constituents of an azeotropic mixture.
In this method a third compound is used in distillation. The process is based on the fact that dehydrating agents like ![]() , diethyl ether etc. depress the partial pressure of one of the original components. As a result, the boiling point of that component is raised sufficiently and thus the other component will distil over.
, diethyl ether etc. depress the partial pressure of one of the original components. As a result, the boiling point of that component is raised sufficiently and thus the other component will distil over.
Dehydrating agents having low boiling point (e.g. ![]() ether) depress the partial pressure of alcohol more than that of water; on the other hand, dehydrating agents having high boiling point (glycerol, glycol) depress the partial pressure of water more than that of alcohol.
ether) depress the partial pressure of alcohol more than that of water; on the other hand, dehydrating agents having high boiling point (glycerol, glycol) depress the partial pressure of water more than that of alcohol.
-
Chromatography
This is a modern method used for the separation of mixtures into its components, purification of compounds and also to test the purity of compounds. The name chromatography is based on the Greek word 'chroma' meaning colour and 'graphy' for writing because the method was first used for the separation of coloured substances found in plants. This method was described by Tswett in 1906.
Principle of chromatography: The technique of chromatography is based on the difference in the rates at which the components of a mixture move through a porous medium (called stationary phase) under the influence of some solvent or gas (called moving phase). Thus, this technique consists of two phases- one is a stationary phase of large surface area while the second is a moving phase which is allowed to move slowly over the stationary phase. The stationary phase is either a solid or a liquid while the moving phase may be a liquid or a gas.
Types of chromatography: Depending upon the nature of the stationary and the mobile phases, the different types of chromatographic techniques commonly used are in a given table,
|
Type of Chromatography |
Mobile/Stationary Phase |
Uses |
|
Adsorption or column chromatography |
Liquid/Solid |
Large scale separations |
|
Thin-layer chromatography |
Liquid/Solid |
Qualitative analysis (identification and characterization of organic compounds) |
|
High performance liquid chromatography |
Liquid/Solid |
Qualitative and quantitative analysis |
|
Gas-liquid chromatography (GLC) |
Gas/Liquid |
Qualitative and quantitative analysis |
|
Partition chromatography or ascending paper chromatography |
Liquid/Liquid |
Qualitative and quantitative analysis of polar organic compounds (sugars, a-amino acids and inorganic compounds) |
It is constant for a given substance (component) under a given set of conditions. Therefore, it is possible to identify the various components by determining their ![]() values.
values.
Refer to the following video for column chromatography:
-
Differential Extraction
This method is used for the separation of an organic compound (solid or liquid) from its aqueous solution by shaking with a suitable solvent (e.g. ether, benzene, chloroform, carbon tetrachloride etc.) in a separating funnel. The selected solvent should be immiscible with water but should dissolve the organic compound to an appreciable extent.
It is important to note that extraction is more efficient (i.e., more complete) when a given volume of the extracting solvent is used in several installments.
This method is normally applied to nonvolatile compounds. For example, benzoic acid can be extracted from its water solution using benzene.
-
Chemical Methods
Besides these physical methods, a number of chemical methods have also been used to separate a mixture of organic compounds. These methods are based upon the distinguishing chemical properties of one class of organic compounds from the others. For example,
Phenols can be separated from carboxylic acids on treatment with an aqueous solution of![]() . Since carboxylic acids dissolve in
. Since carboxylic acids dissolve in ![]() solution evolving
solution evolving ![]() but phenols usually do not react.
but phenols usually do not react.
Destructive distillation of wood gives pyroligneous acid which contains acetic acid (10%), acetone (0.5%) and methanol (3%). Acetic acid can be separated from this mixture by treating it with milk of lime when acetic acid forms the calcium salt. The reaction mixture on distillation gives a mixture of acetone and methanol (which can be further separated by fractional distillation into individual components as mentioned above) while the calcium salt remains as residue in the flask. The calcium salt is then decomposed with dil HCl and distilled to afford acetic acid.
A mixture of 1o, 2o and 3o amines can be separated using either benzenesulphonyl chloride (Hinsberg's reagent) or diethyl oxalate (Hoffmann's method).
-
Purification of Commercial Benzene
Commercial benzene obtained from coal-tar distillation contains 3-5% thiophene as an impurity which can be removed by extraction with conc. ![]() . This purification is based upon the fact that thiophene undergoes sulphonation much more easily than benzene. Thus, when commercial benzene is shaken with conc.
. This purification is based upon the fact that thiophene undergoes sulphonation much more easily than benzene. Thus, when commercial benzene is shaken with conc. ![]() in a separating funnel, thiophene undergoes sulphonation to form thiophene-2-sulphonic acid which dissolves in conc.
in a separating funnel, thiophene undergoes sulphonation to form thiophene-2-sulphonic acid which dissolves in conc. ![]() while benzene does not.
while benzene does not.
After this treatment, the benzene layer is removed, washed with water to remove unreacted ![]() , dried over anhyd.
, dried over anhyd. ![]() and then distilled to give pure benzene.
and then distilled to give pure benzene.
Absolute alcohol from rectified spirit: The rectified spirit (ethanol : ![]() by weight) is kept over a calculated amount of active quick lime (CaO) for few hours and then refluxed. During this process, water present in rectified spirit combines with CaO to form
by weight) is kept over a calculated amount of active quick lime (CaO) for few hours and then refluxed. During this process, water present in rectified spirit combines with CaO to form ![]() . When the resulting mixture is distilled, absolute alcohol distils over leaving behind,
. When the resulting mixture is distilled, absolute alcohol distils over leaving behind, ![]() .
.
-
Drying of Organic Substances
For solids: Most solids are dried first by pressing them gently between folds of filter papers. Compounds which neither decompose on heating nor melt below 100oC are dried by keeping them in steam or oven maintained at 110oC. Substances, which decompose on heating are dried by keeping them in a vacuum desiccator containing a suitable dehydrating agent like fused ![]() , conc.
, conc. ![]() ,
, ![]() solid KOH or NaOH, etc (desiccant).
solid KOH or NaOH, etc (desiccant).
For liquids: Organic liquids are generally dried by keeping them over night in contact with a dehydrating (desiccating) agent which does not react chemically with the liquid to be dried. Commonly used dehydrating agents are quick lime, anhydrous![]() , fused
, fused ![]() or
or ![]() , metallic sodium or potassium, etc.
, metallic sodium or potassium, etc.
Criteria of purity of organic compounds: The purity of an organic compound can be ascertained by determining its some physical constants like m.p., b.p., specific gravity, refractive index and viscosity. In usual practice, sharp m.p. (in case of solids) and boiling point (in case of liquids) are used as criteria for purity because their determination is feasible in the laboratory. A pure organic solid has a definite and sharp (sudden, rapid and complete) melting point, while an impure substance has a lower and indefinite melting point.
Mixed melting point: The melting point of two thoroughly mixed substances is called mixed melting point. This can also be used for ascertaining the purity of a compound.
The substance, whose purity is to be tested, is mixed with a pure sample of the same compound. The melting point of the mixture is determined. If the melting point of the mixture is sharp and comes out to be the same as that of pure compound, it is sure that the compound under test is pure. On the other hand, if the melting point of the mixture is less than the melting point of the pure compound, the compound in question is not pure.
Refer the below mentioned links to get an immediate solution to all queries on Organic chemistry:
JEE Organic Chemistry Syllabus